Chapter 29
Techniques for Working with Wild Reptiles
Amphibian and Reptile Capture Techniques
Passive Capture Methods
Cover Objects
Terrestrial amphibians and reptiles can often be captured under natural cover objects such as woody debris and logs, which offer a favorable microclimate. Artificial cover objects can be placed in suitable habitats to attract them so that these species may be systematically sampled. For terrestrial salamanders, “cover boards” consisting of untreated lumber should be placed flush with the soil in shaded or moist conditions (Figure 29-1). Cover boards can be of varying sizes, but relatively small, thick boards are most effective for salamanders.1 Cover boards should be anchored with a metal stake if they are placed adjacent to a stream or river, where flooding could occur.2 Tin roofing material or black plastic cover sheets can be used to attract snakes and lizards.3 These materials should be placed in open sunny areas and checked in the early morning during hot times of the year or in early afternoon in winter. Any type of artificial cover should be left undisturbed for 2 to 4 weeks after placement to allow it to weather and attract target animals. Cover objects can be carefully lifted with a snake hook to check for amphibians and reptiles.
Leaf Litter Bags
Leaf litter offers substrate to larval salamanders and their invertebrate prey but can be a limited resource in many streams. Leaf litter bags made from plastic bird netting (1.9-cm mesh size) and packed with aged leaf litter can be used to attract larval salamanders in streams.4 Bags can be made by cutting the net into various sized squares (50 × 50 cm to 90 × 90 cm), adding a handful of leaf litter and a few small stones to weight the bag, and pulling the corners of the square together with a plastic zip tie to create a bag.5 The litter bags should be placed in slow moving pools or meanders within streams and anchored to adjacent vegetation. Researchers can check the bags every 2 to 4 weeks by gently removing them from the stream and placing them directly into a shallow pan along with 3 to 4 cm of stream water. The litter can then be carefully searched for larvae.
Polyvinylchloride Pipe Refugia
Polyvinylchloride (PVC) pipe or large diameter sections of bamboo can be used to attract tree frogs.6–8 Pipes or bamboo can be cut into short lengths and can be placed in the ground or hung vertically on a nail placed on the trunk of a tree (Figure 29-2). The diameter of the pipes may affect the number of captures, but generally pipes of ¾″ to 2″ diameter are used.9,10 Ground pipes should be approximately 1 m long, and one end of the pipe should be cut at an angle so they can easily be inserted upright into the soil. Tree pipes should be hung between 1 and 2 m above ground on large diameter trees. Tree pipes can be 0.60 m in length, and placement of a cap on the bottom allows rainwater to accumulate, which makes the pipe more attractive to frogs. A small drain hole should be drilled into capped pipes about 10 cm above the cap to prevent the pipe from completely filling with water. Pipes can be checked by removing them from the ground or tree and checking for tree frogs (Figure 29-3). Frogs can be coaxed from the pipes with the use of a small diameter wooden dowel with a small circular piece of sponge on one end that serves as a plunger. Nontarget taxa (e.g., flying squirrels) may enter the tree pipes and be unable to escape. Thus a short section of nylon rope tied through a hole at the top of the pipe and dangled into the pipe is recommended to allow squirrels a means of escape.11

FIGURE 29-2 Polyvinylchloride (PVC) pipes used to attract tree frogs. Pipes can be mounted on tree trunks (left) or placed in the ground (right).
Active Capture Methods
Netting
A variety of nets and netting techniques are available to capture aquatic amphibians and reptiles; net mesh size and configuration should be based on the size and habit of the species targeted for capture. For most freshwater taxa, sampling with nets should be concentrated in emergent vegetation and other available microhabitats. If netting is conducted systematically, the method can be used to estimate densities of target organisms.12 Sturdy dip nets with a mesh size of 3 mm or less are effective for capturing amphibian larvae and work best in water that is less than 1 m in depth (Figure 29-4). Dip nets should be dragged along the substrate to dislodge and capture amphibian larvae. Seine nets require at least two people but can be used to capture larger amphibians and reptiles in waist deep water. Seine and trammel nets are frequently used to capture Diamondback Terrapins (Malaclemys terrapin) in estuarine systems during mid-ebb through mid-flood tides13 (Figure 29-5).
Hoop Traps
Baited hoop traps are a common method for capturing freshwater turtle species.14 Hoop traps consist of two to three large-diameter hoops enclosed in netting (Memphis Net and Twine Co. Inc., Memphis, Tenn), with a closed end that can be secured above water to a stake or tree and an open end with an inverted funnel that can be anchored to the bottom of a stream or wetland (Figure 29-6). In streams, hoop traps are typically set with the opening pointed downstream, which allows the scent of bait to disperse downstream. In ponds or lakes, hoop traps can be set either perpendicular or parallel to the bank (held in place with two stakes), and the top of the trap should be above water to allow turtles to breathe. A fyke net can be extended from the open end of the hoop trap out into a stream or wetland to guide turtles into the trap. Traps can be baited with chicken livers, sardines, fresh or frozen fish, cat food, canned corn, canned green beans, and watermelon rinds, depending on the diet of the target species.14–16
Basking Traps
Freshwater turtles can be captured using basking traps, which consist of either a wire basket anchored to an existing basking log or a floating artificial basking platform with a net in the center that is placed in a wetland or stream so that turtles are attracted to bask on it. In the latter case, a shallow basket (of varying size depending on the target species) can be made with chicken wire and attached to the side of a basking log with the use of a staple gun or zip ties. The chance of success with this method is maximized if appropriate basking logs can be identified in advance with the use of binoculars. Once a suitable log is identified, the trap should be placed on the downstream side of the log to capture turtles as they attempt to jump into the water away from the boat. Artificial basking platforms can be made with a square floating frame with treadles that can tip the turtles into a barrel or net in the center or that simply relies on the turtles falling into the trap when disturbed.17,18 Artificial basking traps are especially effective when placed amidst floating aquatic vegetation in water bodies with few natural basking sites. Traps can be checked when the sun is high and turtles would be expected to be out basking. Traps should be checked frequently to prevent turtles from overheating.
Drift Fences
A drift fence is a barrier that directs amphibians and reptiles toward traps.19 Fences may be constructed from aluminum flashing, hardware cloth, or siltation fence, a portion of which is buried below ground to prevent animals from digging under the fence. Drift fences can be placed in specific configurations called arrays or may encircle particular habitats, such as a pond, to capture animals migrating to and from a breeding site19,20 (Figure 29-7). Traps are generally placed every 5 to 10 m along a fence or at the ends of short sections of fence. Pitfall (bucket) or funnel traps (described further on) are most effective for terrestrial amphibians, small snakes, lizards, and turtles. Wooden box traps21,22 are effective for capturing large snakes; lizards and terrestrial amphibians can also be captured in box traps along drift fences (Figure 29-8). Traps should be shaded with cover boards, and a wetted sponge or shallow water bowl should be placed in the traps to protect animals from desiccation. This method requires considerable effort to install and maintain, particularly if a wetland is completely encircled. Traps must be checked daily or multiple times a day during migration events and extreme weather conditions (hot or cold) to prevent mortality.

FIGURE 29-7 Drift fence surrounding an isolated wetland in southwestern Georgia. The fence directs amphibians and reptiles toward pitfall traps (19-L plastic buckets) that are shaded and contain a wetted sponge.
Funnel Traps
Various trap designs with inverted funnels at the openings are highly effective at capturing amphibians and reptiles. For example, commercial minnow traps and crayfish traps can be used to capture large aquatic salamanders (Siren spp. and Amphiuma spp.), juvenile and small aquatic turtles, and aquatic or semiaquatic snakes.23 These traps can be placed in standing water with a portion of the trap left above water to allow animals to surface and breathe. Traps should be anchored to a tree or stake so they cannot be dislodged by predators and should be clearly marked so they are easy to find. Several iterations of funnel traps can be made by hand with the use of inexpensive, readily available material. For trapping along a drift fence, funnel traps can be made with the use of aluminum window screen (Figure 29-9). Screen funnel traps can be staked down using a wire pin flag or can be floated in shallow water. A recent variation of a funnel trap is made from a 120-L plastic trash can and funnels made from 6-mm mesh galvanized hardware cloth,24 which was inexpensive to make and highly successful for capturing sirens, amphiumas, and aquatic snakes.
Hook and Line Noose or Cage Traps
Crocodilians can be captured by a hook and line noose or by cage trapping.25,26 The use of a hook and line noose requires close access to the alligator and considerable strength to catch the animal and pull it to shore for further restraint. A treble hook and fishing line can be used to snag the animal on the osteoderms or bony scutes of the dorsum. Capturing individuals greater than 1 or 1.5 m in length generally requires a large (7 to 11) gauge treble hook and doubled 30-lb line with a 6 ft leader and a hook secured with a bimini twist (Andrews K: Pers. observation, 2007). When snagged, the animal can be gradually pulled close enough that a noose can be eased over the snout and tightened, after which the animal can be pulled to shore. Once on shore, the tail can also be noosed (on larger crocodilians), and the jaws and front and hind legs can be restrained with electrical tape. Hook and line techniques can be time-consuming because multiple throws may be required to properly secure the animal, and it may be necessary to allow the animal to become fatigued. However, this technique is the best approach when capturing animals that are close to shore or in habitats inaccessible by boat (e.g., stormwater lagoons). Elsey and Trosclair26 describe large steel traps that can be used for crocodilians; smaller wire-cage live traps (Havahart; Woodstream Corp., Lititz, Pa) can be used for small crocodilians.25
Cage Traps/Bucket Traps for Gopher Tortoises
The two most commonly used methods to trap Gopher Tortoises (Gopherus polyphemus) are bucket traps27 (Figure 29-10) and wire-cage, one-door live traps (Havahart; Woodstream Corp., Lititz, Pa)28 (Figure 29-11). A bucket trap is made with a 5-gallon light-colored plastic bucket with at least ten 0.25″-diameter holes drilled in the bottom. Smaller buckets, or plastic plant pots, can be used to trap juvenile tortoises. The bucket is placed in a hole dug in front of the burrow entrance. Care should be taken when digging the hole because this is typically where female tortoises lay their eggs (eggs may be in the nest from mid-April through October). Newspaper, wax paper, or brown wrapping paper is used to cover the top of the bucket once it has been sunk into the ground. The paper is cut so that it is approximately 2 inches wider on all sides than the diameter of the bucket and secured tightly around the edge of the bucket with a few slits to ensure that it tears as the tortoise steps onto it. A thin layer of sand is placed on the paper to conceal the trap. Shading each bucket trap will prevent trapped tortoises from being exposed to direct sunlight for prolonged periods. Drowning and overheating are the most common problems occurring with this trapping technique. One advantage of this technique over wire-cage traps is that the tortoise can be captured whether it is inside or outside the burrow.

FIGURE 29-10 A, Bucket trap set and ready for trapping. B, Gopher Tortoise (Gopherus polyphemus) caught in a bucket trap (Photos courtesy Tracey D. Tuberville, Savannah River Ecology Laboratory, The University of Georgia, Athens, Ga.)

FIGURE 29-11 A, Scoping a Gopher Tortoise (Gopherus polyphemus) burrow to see if it is inhabited before setting a wire trap. (Photo courtesy Dr. Terry M. Norton, Georgia Sea Turtle Center, Jekyll Island, Ga.) B, Live wire trap, shaded and set in front of Gopher Tortoise burrow. C, Juvenile Gopher Tortoise captured in live wire trap (Photos courtesy Tracey D. Tuberville, Savannah River Ecology Laboratory, The University of Georgia, Athens, Ga.)
An alternative method is using a wire-cage trap in front of the burrow. The burrow should be scoped to ensure that the tortoise is in the burrow before setting a trap. A major advantage of this method is that a hole does not have to be dug; thus there is no chance of damaging eggs if a nest is present, and it is less stressful to the tortoise. Small wire-cage traps can be used to capture juvenile tortoises. The trap should be well camouflaged and shaded. It is advisable to check the traps two to three times per day regardless of type. During the colder months of the year (mid-October through mid-April), tortoises may not leave their burrows for extended periods, which makes these techniques less effective. Tortoises generally will not surface to bask until temperatures reach at least 18°C to 21°C (65°F to 70°F).
Shrimp Trawl
Foraging sea turtles can be collected by independent fishery trawlers. Twenty-meter four-seam nets with 20-cm mesh, without turtle excluder devices, are used by one research vessel to capture sea turtles.29 The trawl duration was limited to 30 minutes (Figure 29-12).

FIGURE 29-12 A, Leatherback Sea Turtle (Dermochelys coriacea) captured with a quick release hoop net. (Photo courtesy of Connie Merigo, New England Aquarium, Large Pelagics Research Center, NMFS Permit #1557-3.) B, Leatherback Sea Turtle on board for tagging, health assessment, and satellite transmitter placement. (Photo courtesy of Kara Dodge, Large Pelagics Research Center, NMFS Permit #1557-3.)
Nesting Beach Encounters
Nesting female sea turtles can be sighted during nightly beach patrols and observed from a distance until they finish nest excavation. Once the turtle starts the process of oviposition, she typically enters a trancelike state and may be safely processed without disrupting normal nesting behavior (Figure 29-13). The turtle should be allowed to complete the nesting process and cover her eggs. Procedures that are more time-consuming, such as satellite tag placement, may require additional restraint. Four slotted boards can be interlocked to create a box around the turtle. Sand can be pushed around the box for further security (Figure 29-14).
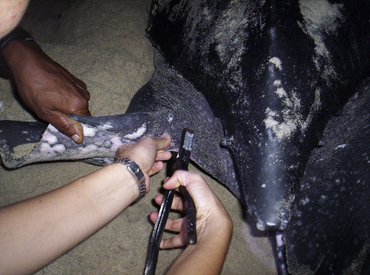
FIGURE 29-13 Female Leatherback Sea Turtle (Dermochelys coriacea) being tagged while laying eggs (Photo courtesy St. Kitts Sea Turtle Monitoring Network.)
Hoop Net and Boat Approach
Foraging Leatherback Sea Turtles (Dermochelys coriacea) can be located via aerial surveys and captured from a boat with the use of a 1.5-m diameter breakaway hoop net. Once secured in the net, the turtle is brought aboard the capture boat for processing. Injury to the turtle can be avoided if the boat is equipped with rubberized surfaces and rounded edges, and air-filled plastic or other similar padding should be placed around the turtles to cushion the body. A continuous flow of sea water over the turtle will help keep it cool while on deck (Figure 29-15).
Handling
Recommended Precautions to Prevent Disease and Other Health Problems
Infectious diseases can be transmitted within and between amphibian and reptile populations when handled for various procedures by field workers. Some examples of significant infectious and parasitic diseases that may be transmitted when appropriate precautions are not taken include chytrid fungus (Batrachochytrium dendrobatidis) in amphibians, iridovirus in amphibians and reptiles, Cryptosporidium serpentis in snakes, paramyxovirus in snakes, Entamoeba invadens in reptiles, Mycoplasma agassizii in tortoises, and herpesvirus (fibropapillomatosis) in sea turtles. The following are some general guidelines for handling and manipulating herpetofauna in the field:
• Avoid using insecticides before handling amphibians and reptiles.
• Wash hands with disinfectant hand wash or wear separate disposable examination gloves after handling each animal.
• Clean any handling equipment with an appropriate disinfectant between each animal.
• Use a separate clean, well-ventilated container or bag for each animal if temporary containment or transport is needed.
• Avoid high or low temperatures during transport of the animal.
• Do not handle or transport amphibians and reptiles from different populations at the same time.
• If wild amphibians or reptiles are held in captivity for various procedures such as surgical placement of radio transmitters, each animal should be isolated in a well-ventilated container and kept at its preferred optimal temperature zone with a thermogradient. The enclosure should be easily disinfected. Wood enclosures should be avoided because they cannot be disinfected.
Marking
Recommended Precautions to Prevent Disease and Other Health Problems
Amphibians
Because of the permeable nature of the amphibian skin, they are very sensitive to many toxins at levels that are well-tolerated without ill effects by reptiles, birds, and mammals. The amphibian skin is very sensitive to human handling. Soaps, lotions, and natural oils can all irritate or damage the skin. Researchers should wear powderless vinyl or plastic gloves when handling amphibians. Many of the common antiseptics and disinfectants are toxic to amphibians (e.g., surgical scrubs with soaps or detergents, iodine products, chlorhexidine, quaternary ammonium compounds, chlorine, alcohol, and ammonia). For these reasons, surgical, injection, and tag sites should be wiped clean of debris and secretions with clean unchlorinated water or saline before the procedure.30
Temporary or Semipermanent Marking and Identification Methods
Photo Identification
Photographs are frequently taken as a form of identification in amphibians and reptiles and can be particularly useful in the case of a morphologic anomaly or injury. High-resolution digital photographs that clearly show the anomaly, injury, mark, or natural pattern of individuals can be taken each time the animal is captured. Photos can be stored electronically or printed and used in the field. Although noninvasive, this method can be time-consuming for identifying individuals and requires that patterns are stable throughout the life of the animal. Thus photographs are most useful as a secondary means of identifying individuals. Some amphibians (e.g., Eastern Tiger Salamander, Ambystoma tigrinum) do not develop spots until several months after metamorphosis, and spots may change slightly as the animal ages. However, with careful attention, patterns can be clearly recognized even after several years (Figure 29-16). Photo identification also has utility for monitoring freshwater, marine, and terrestrial turtles, in which shell anomalies and injuries are common (Figure 29-17). Photographs of the pineal spot of Leatherback Sea Turtles can be useful for later identification.31 This technique can also be useful with patterned snakes, such as rattlesnakes, that have variation in the number or shape of dorsal markings.

FIGURE 29-16 Spot patterns on the dorsum of an Eastern Tiger Salamander (Ambystoma tigrinum). The upper photograph was taken in 2007 and the lower photograph was taken in 2011. White arrows indicate unique spots that can be used to recognize this individual. The red arrows show a slight change in one of the spots over the 5-year period.
Paint Marking
Paint marking has been used as a noninvasive means of identifying reptiles. Different color combinations and paint locations can yield numerous individual marks. Small daubs of model airplane paint applied to the limbs, base of the tail, and dorsum have been recommended for temporarily marking lizards.32,33 Data suggest that paint marking did not increase mortality of lizards from predation34; however, care should be taken to use the minimum amount of paint necessary to identify the lizard. Paint marking has limited utility for most snake species because recaptures are so infrequent. However, paint has been widely used to temporarily mark the rattles on crotalids.35 This technique is particularly useful for capture–mark–release programs in which thorough visual searches for snakes are conducted over short time frames. In this case, recently captured individuals can be identified visually without having to have them in hand. An individual marking scheme can be developed based on color and the position of the marks on the rattle, or a common mark can be used for a cohort or population. The mark can be lost completely if the rattles break; therefore this technique is most useful for identifying individuals within an activity season.
Paint pens and nontoxic nail polish have been useful in temporarily marking the carapace of a variety of freshwater aquatic and terrestrial chelonian species (Figure 29-18). One example in which this is particularly useful is for identifying individual Gopher Tortoises in their burrow with a burrow camera scope. If the tortoise had already been processed that season, it had a number on its carapace that was readily visible with the scope. This information is essential when setting traps to capture these tortoises (Figure 29-19).

FIGURE 29-18 Gopher Tortoise (Gopherus polyphemus) hatchlings marked with a paint pen and nail polish.

FIGURE 29-19 Adult Gopher Tortoise (Gopherus polyphemus) with paint pen number and radio transmitter on carapace going into a burrow.
Paint and other temporary marks have also been applied to sea turtles, but the technique is less common than in freshwater and terrestrial turtles. Because of the wear on the carapace and the corrosive nature of salt water, paints do not last as long as in freshwater. Two-part resins have also been used to temporarily mark sea turtles.36,37 To further increase retention throughout a nesting season, one can use a Dremel tool to create grooves in scutes that can then be filled with paint.36 Paint, like other temporary marking techniques, is generally used in conjunction with other more permanent marking techniques.38
Colored Beads
Small colored and numbered beads have been used to mark American Alligators ([Alligator mississippiensis]; Tuberville T: Pers. com., Jan 14, 2012; [Figure 29-20]) and West Indian Rock Iguanas (Cyclura spp.; Hudson R: Pers. com.; [Figure 29-21]). In the alligator study, researchers applied two beaded tags on the single row of tail scutes using fishing line. Using two tags that varied in color and number, they were able to identify individuals and to differentiate among experimental groups without having to handle the animals. Marking could involve one to three beads of varying colors and numbers on each individual. Bead retention was sufficiently high in captive animals, but this marking technique would presumably not be viable in the wild where interaction with other animals and habitat debris would decrease retention rates.

FIGURE 29-20 Photograph of colored and numbered beads used to mark American Alligators (Alligator mississippiensis). (Photo courtesy Tracey D. Tuberville, Savannah River Ecology Laboratory, The University of Georgia, Athens, Ga.)
Fluorescent Polymers, Pigments, and Tags
Acrylic polymers (also called visible implant elastomers [VIEs]) and fluorescent pigments can be injected into the skin of amphibians in different portions of the body (e.g., the tail of salamanders, sides of the body, and hind legs of frogs). Different injection sites, color combinations, and numbers of marks can allow for large numbers of individuals to be uniquely marked, and marks can be retained for up to 2 years.39 This technique is one of the few reliable methods for marking larval amphibians; polymers can be injected into the tail fins of larvae.40
Small prenumbered fluorescent tags (Figure 29-22); Northwest Marine Technology, Inc., Shaw Island, Wash; Floy Tag, Inc., Seattle, Wash) also can be implanted subcutaneously in frogs and salamanders.41 These tags come in a variety of colors and sizes and work best in species with lightly pigmented skin. These fluorescent tags can be accurately read using an LED light.42 Floy tags have been used as tertiary identification in some chelonian research projects. The numbered tags can be secured to the shell with a clear, fast-curing epoxy. As stated earlier, the tags come in a variety of sizes and are small enough to use on juveniles without compromising their growth.
Monel, Inconel, and Cattle Ear Tags
Monel and cattle ear tags are effective and an inexpensive means of marking crocodilians. Monel tags have been attached to the webbing on the rear foot of American Alligators43; this technique requires recapturing the animal to read the tag. Monel tags do not corrode as rapidly in freshwater systems as they do in salt water (see further on); thus these tags are effective for most crocodilians. Plastic cattle ear tags have also been used to mark crocodilians. Roto (Sheepman Supply Co., Frederick, Md) and Allflex (Allflex USA, Inc., Dallas, Tex) ear tags, in particular, are inexpensive. These tags experience a higher rate of retention when the scute is pierced on the tail of American Alligators prior to attachment, by using a fine drill bit on the tagging device to facilitate penetration and secure latching of the male and female components of the tag (Andrews K: 2010). However, retention of external tags is generally low in crocodilians because of extensive, aggressive interactions with conspecifics. The use of these tags is beneficial because individuals can be identified from a distance with binoculars when the animal is out of the water.
Both plastic and metal tags are used to mark the flippers of sea turtles. Tags come in varying sizes, and smaller tags should be used on immature turtles. Plastic tags are inexpensive but are susceptible to breakage, wear, and entanglement in nets.36 Although tags made from metal alloys (Monel, Inconel, and titanium) may last longer than plastic tags, these tags are more expensive than plastic tags and Monel tags can also be susceptible to breakage or corrosion, which can lead to infection at the attachment site.44 Research entities such as the Archie Carr Center for Sea Turtle Research at the University of Florida and the Wider Caribbean Sea Turtle Network (WIDECAST) work in coordination to facilitate a global database of turtle tags and to reduce potential redundancy in marking. In addition to individual marking codes, contact information can be placed on the back tags to assist with tag recoveries.
In sea turtles, tags are generally attached to the edge of fore flippers, and when they are applied correctly, the risk of infection or injury at the attachment site is generally low. Tags may either be inserted through the keratinized scales on the flippers or in the soft tissue between scales. Although no comparative data are available, tags placed in the middle of the scales have been retained for approximately 20 years in some individuals.36,45 Tags are typically placed either in the most proximal pad on the posterior edge of the fore flipper or on the second pad to prevent the turtle from biting the tag46 and to reduce drag or contact with other animals (Figure 29-23).47 If the original tag is lost, replacement tags should be placed in a different pad rather than over the scar from the initial tag. Researchers generally tag both foreflippers, but the proximal pad of rear flippers are frequently tagged on juveniles and Leatherback Sea Turtles.36
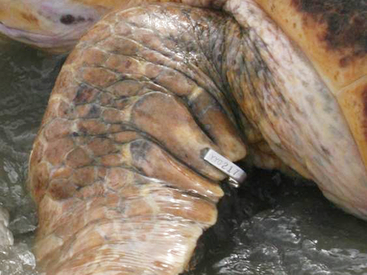
FIGURE 29-23 Placement of metal Inconel flipper tags on proximal pads on the front flipper of a marine turtle; the curved part of the tag allows room between the tag and flipper to reduce risk of constriction. (Photo courtesy of S. Dilts.)
Many of the factors affecting retention are avoidable through proper application and the incorporation of aseptic techniques to reduce risk to turtle health. A common cause of tag loss is failure of the locking mechanism44; tags should be double-checked before release and replaced if necessary. Applicators should be cleaned regularly to reduce rust and debris that can impede proper application of the tag, and they should be replaced when necessary to avoid tag loss. Tags may also be lost if they are snagged by debris (e.g., nets)44 or substrate, as is the case with Hawksbill Sea Turtles (Eretmochelys imbricata), which shelter in coral reefs. Tearing generally results in tag scars that are readily visible and provide some information on whether the animal was marked in the past. Flipper tags can also be lost because of tissue necrosis if they are improperly applied. Tags should be placed such that roughly two thirds of the tag covers the flipper and the remaining one third, specifically the curved part, hangs over the edge of the fore flipper so that it does not constrict circulation or growth. Because of problems with tag retention, biologists regularly tag both fore flippers in addition to using permanent marking methods such as PIT tagging. It is imperative to account for flipper tag loss to avoid overestimation in population abundance and underestimation of survivorship.48
Permanent Marking Methods
Passive Integrated Transponder Tags
Passive radio frequency identification (RFID) tags (or PIT tags) are one of the most widely used and reliable tools for marking amphibians and reptiles.49,50 A PIT tag is an electronic microchip encased in biocompatible glass that ranges in size from 8 to 22 mm long and about 2 mm in diameter; the smallest tags weigh less than 0.10 g (Figure 29-24). Tags are dormant until activated by a handheld reader (Figure 29-25), which generates a close-range, electromagnetic field. Each tag contains a unique alphanumeric code that allows an indefinite number of individuals to be marked. The detection range for PIT tags varies with the size of the tag and type of reader. PIT tags can be expensive when large numbers of individuals are marked but have the benefit of small size and a potentially infinite life span.
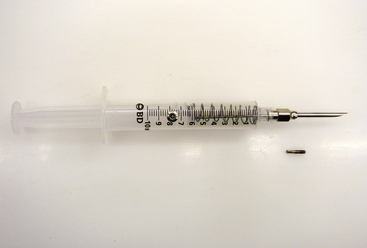
FIGURE 29-24 Syringe and passive integrated transponder (PIT) tag used to mark amphibians and reptiles for individual recognition. Tags are passive (no battery).

FIGURE 29-25 The unique alphanumeric code of a passive integrated transponder (PIT) tag is activated when scanned by a manufacturer-specific PIT tag reader. (Photo courtesy of D.E. Scott.)
There are two mainstream manufacturers of PIT tags: EZid American Veterinary Identification Devices (AVID, Weld, Colo) and Biomark, Inc. (Boise, Idaho), although there are additional manufacturers.50 Both companies manufacture tags that are reliable and functional for the life of most animals, provided tags are inserted correctly (see further on); however, data on retention in long-lived species are not yet available. Biomark offers waterproof readers that are durable for use in the field. It is best to use one supplier at a field site because some brands are encrypted and cannot be read with the readers of other manufacturers. Further, care should be taken when recording identification codes from the reader because it is easy to accidentally transpose numbers or letters.
In addition to marking individuals, PIT tags can be used to monitor thermoregulatory behavior and movements of animals. BioMedic Data Systems, Inc. (Seaford, Del) produces temperature-sensitive PIT tags that have been used to monitor body temperature in snakes.51,52 Automated scanning systems placed in areas the animal is expected to cross can be used to track movements of PIT-tagged animals. Scanning systems have been used to monitor migrations of PIT-tagged amphibians to and from breeding ponds or burrows53–55 and use of passages and culverts under roads by Desert Tortoises (Gopherus agassizii).56 However, scanning systems are expensive, and the detection range of PIT tags in the field can be very limited.
PIT tags are injected or inserted through a surgical incision, most commonly under the skin, into muscle or the body cavity (Figure 29-26). Application of tissue glue at the insertion site can help with tag retention and can control bleeding (Norton T: Pers. observation, 2011). Tags can be purchased in preloaded sterile syringes or individually and loaded into a sterilized stainless steel syringe for implantation (see Figure 29-24). All PIT tags should be sterilized before insertion if this has not been done by the commercial source. The preferred technique for sterilization is ethylene oxide gas (STERIS Isomedix Services, Mentor, Ohio) or hydrogen peroxide plasma sterilization (Sterrad, Advanced Sterilization Products, Irvine, Calif). Sterility should be maintained during the loading and insertion of the PIT tag.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree















