Chapter 4. Surgical trauma and iatrogenic lesions
CHAPTER CONTENTS
COMPLICATIONS OF INTRAOCULAR SURGERY
Of submissions in the COPLOW archive, 1.3% are related to complications of surgery.
General categories of surgical complications
Intraoperative complications leading to submission of specimens to the pathology laboratory (Fig. 4.1):
• Expulsive choroidal hemorrhage (Fig. 4.1)
▪ Rapid hemorrhage in the suprachoroidal space in an open, decompressed globe causes expulsive anterior displacement of ocular tissues, and is a devastating complication
– This is associated with the sudden onset of ocular hypotony
– In the COPLOW collection, this is often seen in association with acute non-surgical trauma. This could reflect a failure to submit surgical cases, or may indicate that this is a rare complication of intraocular surgery in animals
– Fortunately, when this is seen in the COPLOW collection as a surgical complication, it is usually in eyes where the sclera was inadvertently cut during an enucleation procedure
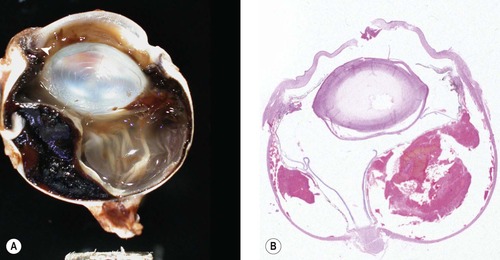 |
| Figure 4.1 Acute trauma, expulsive choroidal hemorrhage. (A) Gross photograph of a canine globe with a penetrating traumatic injury (arrow) and expulsive hemorrhage in the choroid. (B) Subgross photomicrograph of a canine eye showing expulsive choroidal hemorrhage, which occurs when the pressure in the globe rapidly decreases. |
• Evisceration procedures aborted due to unanticipated findings or procedural complications
▪ The COPLOW collection contains 29 cases where evisceration surgery has been aborted and enucleation of the partially collapsed globe performed
– All but three of these submissions were from evisceration procedures aborted by the surgeon because evidence of neoplastic or active inflammatory processes was encountered.
Inflammation associated with known or presumed infection or toxic contamination during surgery (Figure 4.2, Figure 4.3 and Figure 4.4)
• ‘Outbreaks’ of endophthalmitis in multiple consecutive, or nearly consecutive, cases from the same practice
▪ COPLOW has examined a series of cases from three such outbreaks
– Contamination or manufacturing defects in viscoelastic (one series) and prosthetic intraocular lenses (two series) were implicated as sources of intraocular contamination
– Endophthalmitis was recognized clinically within a few days of the surgical procedure and progressed rapidly
– Suppurative, neutrophilic endophthalmitis centered on the anterior segment
– Delayed healing of the surgical incision and suture track inflammation were seen in several of the globes. In these cases, incisional abnormalities were assumed to be secondary to endophthalmitis. However, as definitive cause-and-effect relationship could not be established, incisional complications may also have directly contributed to the development of endophthalmitis
– Only a single case from one of the series had histologically demonstrable bacteria, identified in the vitreous near the posterior lens capsule
• Sporadic endophthalmitis following intraocular surgery (Fig. 4.2)
▪ There are 40 cases in the COPLOW database
▪ Affected globes were typically enucleated between 3 days and 3 months postoperatively
– Those cases, in which enucleation occurred within 7 days of intraocular surgery, had intraocular sepsis, based on direct observation of bacterial organisms
– The cases in which enucleation took place more than seven days postoperatively seldom had demonstrable sepsis
▪ These cases often had evidence of severe corneal pathology, that included (Figs 4.3, 4.4):
– Dehiscence of the surgical incision
– Suture tract suppuration
– Stromal abscessation
– Perforation away from the surgical wound.
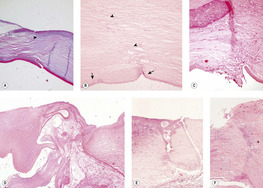 |
| Figure 4.3 Complications of the corneal surgical incision. (A,B) Photomicrographs showing the keratotomy site in canine cataract surgery without complications from the surgical incision (arrowheads). The small arrows point to the ends of a break in Descemet’s membrane. (C) Photomicrograph of the inner aspect of a canine cataract surgery corneal incision showing mild fibroblast proliferation, vascularization, and corneal epithelial down-growth. (D–F) Three photomicrographs of cataract surgery corneal wounds. Varying degrees of suppurative inflammation are severe enough to cause intraocular complications or wound dehiscence. |
 |
| Figure 4.4 Collagenolysis. (A) Gross photograph of a canine globe with collagenolysis of the axial corneal stroma. (B) Photomicrograph of a canine globe with a mid-stromal abscess. (C) Acute collagenolysis (*) in an affected canine cornea. |
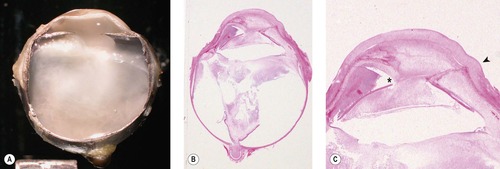 |
| Figure 4.2 Postoperative inflammation. (A) Gross photograph of severe endophthalmitis/vitreitis, which occurred after cataract surgery in a dog. (B,C) Subgross and low magnification photomicrographs of the same canine globe show suppurative endophthalmitis and posterior synechia (*). The arrow in (C) points to the surgical incision. |
Long-term postoperative complications (manifesting long after the surgery) (Figure 4.5, Figure 4.6, Figure 4.7, Figure 4.8 and Figure 4.9)
• Implantation of corneal or conjunctival epithelium, within the corneal stroma leading to the formation of inclusion cysts (Fig. 4.5)
▪ Epithelial inclusion cysts present as focal, opaque nodules in the cornea at the site of surgery or other trauma (see also Ch. 8)
▪ Histologically, inclusion cysts are bland, localized nodules that have a lining of fully-differentiated epithelium and are often filled with keratin or mucin
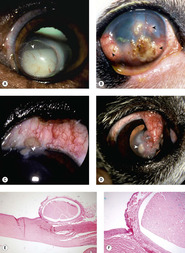 |
| Figure 4.5 Epithelial inclusion cysts. (A) German Shorthaired Pointer, 6 years old: following trauma, this large cyst developed. Epithelial cells can be seen settling in the cyst (arrow). (B) Boston Terrier, 3 years old: multiple inclusion cysts are present (arrows) following a grid keratotomy. (C) German Shepherd Dog, 12 years old: limbal inclusion cysts are present (arrow) following a limbal incision for lens extraction. (D) Boston Terrier, 9 years old: the cyst (arrow) formed following the placement of a conjunctival flap. (E,F) Photomicrographs of an epithelial inclusion cyst embedded in the substantia propria of canine limbal conjunctiva. |
• Corneal edema associated with endothelial damage
▪ Corneal edema associated with compromised endothelial function causes pronounced stromal thickening, a blue/white corneal opacity, and an increased susceptibility to infection as well as an increased risk of collagenolysis
▪ Intraocular surgery is a major hazard to the corneal endothelium, which has limited or no regenerative potential in adults
▪ Direct mechanical contact can be harmful to the endothelium
▪ Separation of the endothelium from the stroma during surgery can also be harmful
▪ Exposure to irrigating solutions, viscoelastic materials (used to protect the endothelium and help maintain the anterior chamber intraoperatively), or other pharmacologic preparations can be harmful to the endothelium, particularly if contaminated with endotoxin, or if contact with the endothelium is prolonged. Permanent damage to the endothelium is unlikely when irrigation solutions such as saline or balanced salt solution are used in limited volumes (100 mL or less) over limited periods of time
▪ Morphologic changes seen in the corneal endothelium after intraocular surgery include (Fig. 4.6):
– Attenuation
– Spindle cell metaplasia and retrocorneal membrane formation
– Duplication of Descemet’s membrane
○ The mechanism of this duplication is not clear, but it is commonly observed after surgery or blunt trauma. Endothelial separation and reattachment may induce duplication of Descemet’s membrane
○ This morphologic feature may also be seen following non-surgical trauma (discussed in Ch. 5).
– Multiple breaks in Descemet’s membrane (striate keratopathy)
○ Manipulation and bending of the cornea during surgery can cause Descemet’s membrane to rupture
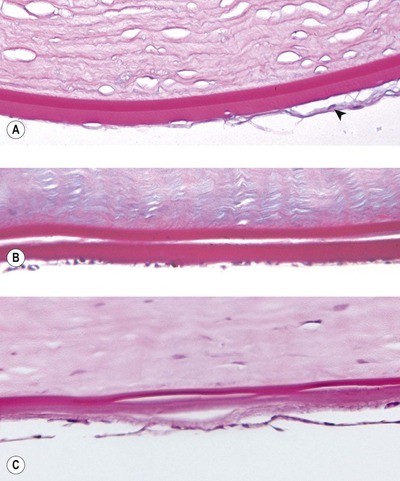 |
| Figure 4.6 Corneal endothelial and Descemet’s membrane changes. (A) Photomicrograph showing a Descemet’s membrane with distinct anterior and posterior components and a retrocorneal membrane (arrow) consisting of spindle cells, which replace the native endothelium. (B) Photomicrograph showing complete duplication of the Descemet’s membrane. The endothelium is still recognizable but attenuated. (C) Descemet’s membrane has a variable thickness and blends with a retrocorneal membrane anterior to the attenuated endothelium (PAS stain). |
• Intravitreal traction bands and retinal detachment
▪ Disruption of the vitreous and low-grade inflammation in the globe lead to fibrous bands (traction bands) across the anterior vitreous and subsequent retinal detachment (Fig. 4.7)
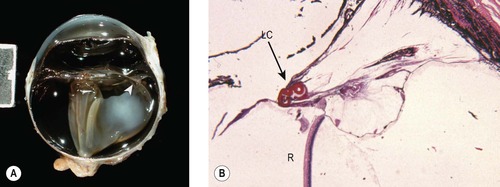 |
| Figure 4.7 Retinal detachment. (A) Gross photograph of a canine globe showing retinal detachment associated with traction bands (arrow) between the lens capsular bag and the peripheral retina. (B) Low magnification photomicrograph showing the peripheral retina (R) pulled toward the lens capsular bag (LC and arrow) because of a contracting spindle cell membrane (PAS stain). |
▪ Vitreal traction bands are more likely to occur if there is inflammation or hemorrhage in the postoperative period
• Glaucoma (Fig. 4.8)
▪ Neovascular glaucoma
– Retinal detachment is associated with the release of the growth factors, vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF). Soluble VEGF, in particular, stimulates pre-iridal fibrovascular membrane formation. This, in turn can cause peripheral anterior synechiae and the development of neovascular glaucoma
▪ Glaucoma secondary to posterior synechiae (iris bombé)
▪ Glaucoma secondary to anterior synechiae
▪ ‘Malignant glaucoma’ may occur in the immediate postoperative period, or as a longer-term complication. This may result from disruption and posterior misdirection of aqueous humor flow by anteriorly prolapsed vitreous, and/or inflammatory membranes extending across the anterior vitreous face, ciliary body (cyclitic membranes) or pupil, in eyes following lens or cataract extraction
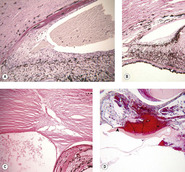 |
| Figure 4.8 Glaucoma. (A,B) Photomicrographs of canine iris and iridocorneal angle show pre-iridal fibrovascular membrane and peripheral anterior synechia leading to neovascular glaucoma. (C) Photomicrograph of a dog eye showing anterior synechia at the surgical scar contributing to angle closure. (D) Low magnification photomicrograph of a dog eye showing hemorrhage and posterior synechia between the iris and the lens capsular bag (arrow) causing pupillary block. |
• Epithelial down-growth and epithelial lining of the globe (Fig. 4.9)
▪ The surface epithelium can gain entry to the globe through the surgical incision or through suture tracts. In many instances, its presence signals poor incision closure technique
▪ Once stratified squamous epithelium or conjunctival epithelium is introduced into the globe, it can slowly cover the internal surfaces of the ocular tissues, interfering with their function, stimulating inflammation in response to released keratin, or obstructing aqueous flow
▪ Entrapped fragments of epithelium can become cystic within the corneal or scleral stroma, forming epithelial inclusion cysts.
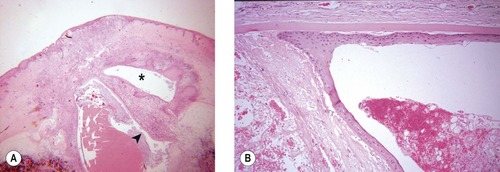 |
| Figure 4.9 Epithelial down-growth. (A) Photomicrograph of a dog eye showing severe corneal inflammation at the surgical incision, with granulation tissue and epithelial down-growth that form a pocket within the deep stroma (*) and also within the anterior chamber (arrow). (B) Photomicrograph of a dog eye with fully-differentiated stratified squamous epithelium in the anterior chamber associated with pre-iridal fibrovascular membrane and broad anterior synechia. |
Comparative Comments
A greater proportion of human eyes submitted for pathology have had intraocular surgery as compared with the COPLOW eyes. Few of these human eyes, however, are submitted because of direct complications of intraocular surgery. Rather, in human eyes, multiple operations are commonly done to avoid blindness following non-surgical trauma or to prevent further vision loss in conditions such as glaucoma, retinal detachment, or retinal vascular disease. Expulsive choroidal hemorrhages, endophthalmitis, corneal decompensation, retinal detachment, glaucoma, and epithelial in-growth are all feared complications of intraocular surgery that are encountered in human specimens as well as in animals.
THE FULL-THICKNESS CORNEAL INCISION AND ITS VARIATIONS
Morphologic features of the uncomplicated corneal incision (Fig. 4.10)
• In the first few weeks after surgery, a granulomatous response to suture material within cornea may be seen. Suppurative response and epithelialization of suture tracts may be greater with absorbable (e.g. polyglactin 910) than with non-absorbable (e.g. monofilament nylon) suture materials
• With good apposition, there is minimal scar formation in the corneal stroma, and recognizing the incision site is difficult, unless the submitting clinician clearly identifies its location. Below are clues to identifying the healed, uncomplicated surgical wound:
▪ Identify the break in Descemet’s membrane
– In a surgical incision or traumatic rupture of the cornea, there is often recoil of the membrane which causes it to have curved edges
▪ Examine the corneal surface, looking for any evidence of disruption to the epithelial basal lamina, or of epithelial down-growth
▪ Using low magnification, look for a full-thickness, lineal disruption in the regular, lamellar structure of the normal corneal stroma.
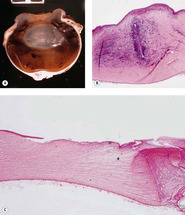
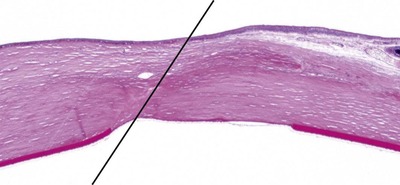 |
| Figure 4.10 Uncomplicated corneal incision. Photomicrograph showing an uncomplicated corneal incision scar in a canine cornea indicated by the line. |
Iridal entrapment or prolapse (Fig. 4.11)
• Due to the hypotonic nature of the opened globe that leads to anterior displacement of iris tissue, or because the iris was inadvertently pulled into the incision during closure, it is not unusual to find iris tissue entrapped in the incision. Subsequently, these uveal remnants will become incarcerated in the incisional scar tissue
▪ This entrapment causes delayed or imperfect wound healing, focal corneal opacity and anterior synechiae. The latter may contribute to postoperative glaucoma if extensive.
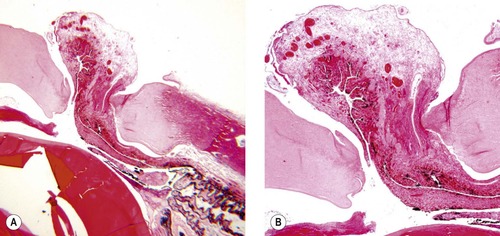 |
| Figure 4.11 Corneal perforation and iris prolapse. (A,B) Photomicrographs of the same dog eye viewed at different magnifications show iris prolapse into the surgical incision causing acute surgical wound dehiscence. |
Adverse reactions around sutures (Fig. 4.12)
• Epithelial down-growth
• Inflammation.
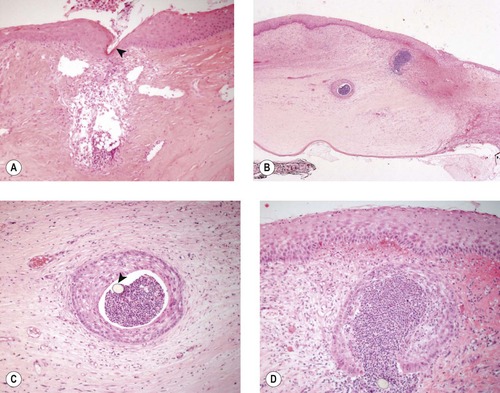 |
| Figure 4.12 Epithelial down-growth and inflammation around sutures. (A) Photomicrograph showing suppurative inflammation in the corneal stroma where the suture (arrow) penetrates the cornea. (B) Low magnification photomicrograph showing epithelial down-growth and suppurative inflammation in a suture track. (C,D) Higher magnification photomicrographs show the same suture track as in (B). The arrow points to the monofilament suture in (C). |
Wound dehiscence
• Dehiscence of the surgical incision is usually seen in conjunction with inflammation
• Consequences of dehiscence of the corneal incision
▪ Delayed healing of the incision
▪ Weakened surgical scar
▪ Corneal opacity
▪ Introduction of sepsis leading to endophthalmitis.
Aspiration or injection sites (Fig. 4.13)
• Injection or aspiration sites are often sampled as part of the protocol in studies involving intraocular injection or aspiration
• Although properly performed injections or aspirations have a low rate of complications, no procedure is entirely ‘safe’. It is instructive to look at the local tissue reactions that occur at the site of such a common and, seemingly, innocuous procedure
• Lesions that may be seen at aspiration or injection sites include:
▪ Disruption of scleral collagen
▪ Phagocytosis of hypodermic needle lubricant, recognized as refractile, non-staining material
▪ Glial and spindle cell proliferation within the vitreous
▪ Vitreous prolapse into the site (this is more likely with aspiration techniques)
▪ Epithelial down-growth.
Comparative Comments
Following the advent of microscopic surgery, iris incarceration or prolapse, suture problems, and wound dehiscence are extremely rare occurrences in human eyes submitted to the pathology laboratory.
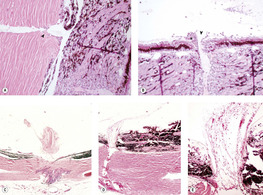 |
| Figure 4.13 Injection and aspiration sites. (A) Photomicrograph showing the scleral aspect of a pars plana injection site in a primate eye. Small numbers of bland macrophage cells are seen within the cavitated area of the sclera (arrow). (B) Photomicrograph showing the ciliary body aspect of the same injection site as (A). There is a defect in the ciliary body epithelium (arrow) and an early reactive proliferation of spindle cells and glial cells (*). (C) Photomicrograph of a pars plana injection site in a canine globe showing an inward proliferation of spindle cells and/or glial cells. (D,E) Aspiration site in a canine globe. In this case, the aspiration was performed immediately before enucleation, but vitreous pulled into the aspiration defect might have complicated healing. |
TISSUE EFFECTS OF ELECTROCAUTERY, CRYOSURGICAL AND LASER APPLICATIONS
The effects of electrocautery (Fig. 4.14)
• Used for surgical cutting and hemostasis
• Electrocautery creates a tissue artifact that the pathologist needs to be aware of when interpreting histopathological findings
▪ Electrocautery affects the tissue immediately in contact with the device, regardless of the tissue type
▪ These effects are seen at surgical margins where electrical energy was used for cutting or hemostasis
▪ Electrocautery imparts a coagulation effect on connective tissues, seemingly fusing the collagen and other protein structures
▪ The effect on epithelia is to cause cells to elongate into a distorted, spindle cell profile.
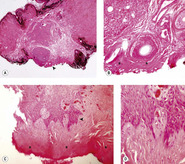 |
| Figure 4.14 Tissue artifacts resulting from electrocautery. (A,B) Low magnification photomicrograph (A) and higher magnification (B), of the area by the arrow, show coagulated tissue (*) typical of electrocautery. (C,D) Low magnification photomicrograph (C) showing electrocautery burn similar to (A) and (B) (*). The higher magnification photomicrograph (D) showing, from the area near the arrow, the stretching effect of electrocautery on epithelial cells. |
The effects of cryotherapy
• Used for focal ablation of ciliary body tissue in glaucoma; for retinopexy in the treatment or prevention of retinal detachment; treatment of mass lesions, including corneal, limbal and adnexal neoplasms, and in the management of distichiasis. Cryogens used in ophthalmic practice include liquid nitrogen, nitrous oxide and carbon dioxide. The advantages and disadvantages of different cryogens and cryosurgical instruments are important considerations in their selection for specific ophthalmic applications
• Cryotherapy affects a sphere of tissue within a radius extending from the application device
• Freezing causes cell lysis, cellular dehydration and thermal shock with ischemic/and coagulation necrosis, with subsequent removal of the affected cellular components. Freezing has little effect on the qualitative nature of the connective tissues, such as the sclera. Eventually stromal elements and epithelium may ‘grow back’, but return of more complex tissue organization is incomplete
• The acute effects of cryotherapy are tissue edema and necrosis which stimulates a granulation tissue response
• The end effects are atrophy and disorganization of the affected tissues.
The effects of surgical laser (Figs 4.15, 4.16)
Diode lasers
• Penetrate deeply into the tissues until preferentially absorbed by pigmented tissue, when their energy is converted to heat
• May be delivered through the clear cornea without causing significant damage to the corneal endothelium
• Morphologic consequences of diode laser use:




▪ The effect occurs by thermal coagulation of tissues within a certain radius of where the heat energy is generated (usually pigmented tissue)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


