Chapter 9 Surgical procedures of the anterior chamber and anterior uvea
Introduction
Leukocytic infiltration of the anterior uvea also affects the anterior chamber, causing the aqueous humor to become cloudy (‘aqueous flare’ – Tyndall effect), with gravitation of cells in the ventral anterior chamber producing hypopyon and the adherence of inflammatory cells on the posterior cornea (keratic precipitates). Anterior uveal inflammations in cats are usually associated with systemic diseases, and frequently require additional diagnostic procedures to establish the diagnosis (Fig. 9.1). Anterior chamber paracentesis (keratocentesis) with cytologic and protein (globulin, albumin, antibodies, etc.) analysis may assist in establishing the diagnosis.
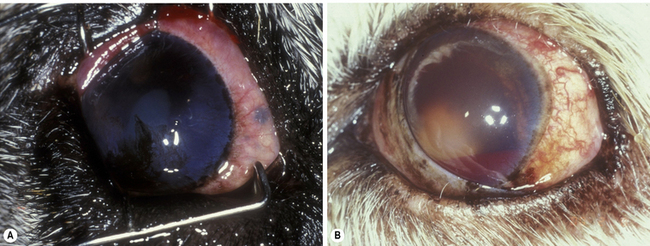
Anterior uveal neoplasms are frequent in small animals, but less frequent in horses. Clinical differences in the malignancy of these tumors in the dog and cat necessitate different strategies for their clinical management. Anterior uveal neoplasms in the dog are primarily malignant melanomas and, less frequently, ciliary body adenomas and adenocarcinomas (Fig. 9.2). These neoplasms, affecting the iris, ciliary body or both structures, usually enlarge slowly and metastasize late and infrequently (about 5%). The clinical history of these neoplasms usually includes local irritation, ocular inflammation, and enlargement of the globe. Space-occupying masses are commonly associated with secondary glaucoma and retinal detachments. Hyphema or hemorrhage in the anterior chamber may result from the rapidly growing tumor, secondary glaucoma and lens luxation, and retinal detachments. Types of neoplasm affecting the anterior uvea of large animals are similar, with melanoma the most common primary tumor.
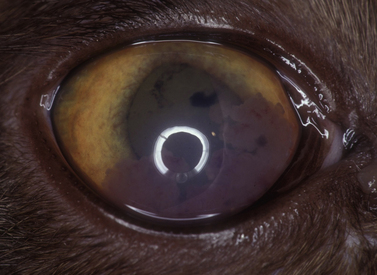
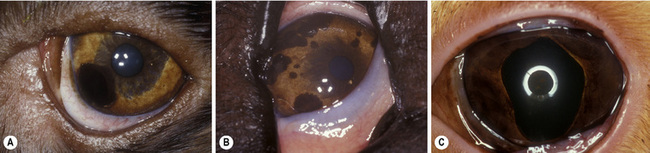
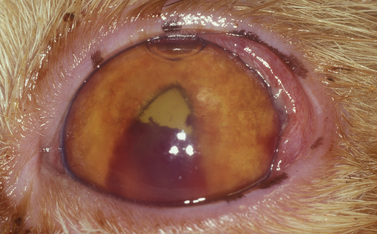
Small isolated iridal neoplasms in dogs and horses may be treated by iridectomy or iridocyclectomy combined with excision of the adjacent sclera, and more recently by diode laser photocoagulation (Fig. 9.3a,b). Diffuse iridal melanomas in cats are managed differently, because the potential for local infiltration and metastasis is greater. Cats with diffuse iridal melanomas are usually presented with a progressive brown to black pigmentation of the iris (Fig. 9.3c). The iridal mass increases in thickness late in the disease. Pupillary changes, secondary glaucoma, hyphema, and retinal detachments indicate that the iridal neoplasm is advanced, and an enucleation should be performed. Controversy exists among veterinary ophthalmologists and veterinary pathologists as to early clinical management of these neoplasms when the only clinical sign is the iridal pigmentation, which is progressing slowly. Diffuse iridal melanomas in cats usually involve the majority of the iris and are not amenable to sectional iridectomy or iridocyclectomy.
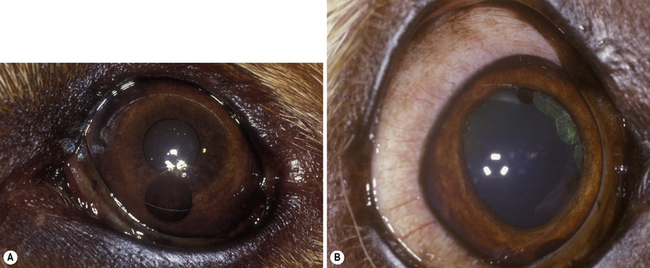
Cysts of the iris and ciliary body are not infrequent in older dogs, cats, and horses (including those of the granula iridica). Iris cysts arise from the posterior iridal epithelium, and appear as densely pigmented, single or multiple spherical bodies. In dogs, they may be free-floating within the anterior chamber, in the pupil, or still attached to the posterior iridal surface (Fig. 9.4). In cats, most iridal cysts remain attached to the pupillary margin. In dogs presented with iridal cysts in the anterior chamber, the drug-induced mydriasis associated with the ophthalmic examination may liberate additional cysts into the anterior chamber that were trapped behind the pupil in the posterior chamber. The free-floating cysts gravitate to the most ventral portion of the anterior chamber. Manipulation of the animal’s head and ocular movements will often cause these bodies to move within the anterior chamber. Sometimes these iridal cysts become trapped in the anterior chamber angle, and may simulate a basal iridal melanoma. These small round black iridal cysts transilluminate, distinguishing them from anterior uveal melanomas. B-scan ultrasonography will also reveal hollow centers. Treatment includes temporization, laser-induced rupture or deflation, and paracentesis or lavage from the anterior chamber.
Surgical anatomy
Limbus
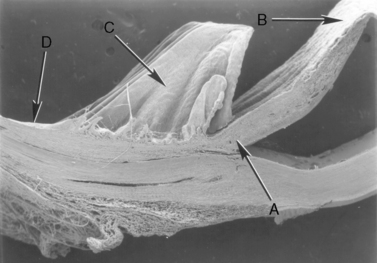
Surgical entry into the anterior chamber usually occurs through the peripheral cornea, limbus, and at the limbal–scleral junction (Fig. 9.5). Most approaches for anterior uvea and glaucoma surgeries enter the anterior chamber at the limbus (‘blue zone’), but may be accompanied by limited hemorrhage. Peripheral corneal incisions are usually used for cataract and lens removals, are performed faster, and preferred by most veterinary ophthalmologists. The limbus–scleral incision is not usually performed because of the resultant hemorrhage but offers the possibility of the least postoperative corneal astigmatism.
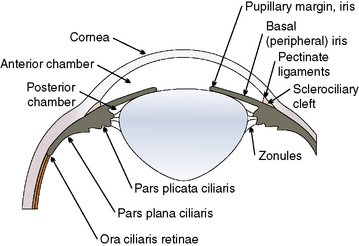
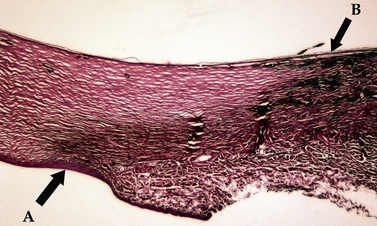
The limbus (‘blue zone’) is the 0.5–1.0 mm junction of the clear cornea and opaque white anterior sclera (Fig. 9.6). The limbus may contain some pigmentation (especially laterally), and a few small blood vessels. On its anterior surface the non-keratinized squamous epithelium of the cornea begins to transform to keratinized squamous epithelium of the bulbar conjunctiva. The limbus prevents direct observation of the anterior chamber angle and the aqueous humor outflow passages into the ciliary or sclerociliary cleft. In this zone regular corneal stroma lamellae begin to form the irregular dense connective tissues of the sclera. Blood vessels, absent in the normal cornea, are present in the anterior scleral tissues next to the limbus.
Iris
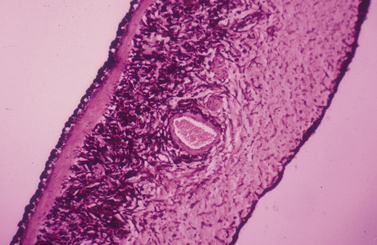
The animal iris is comprised of highly vascular, friable, and spongy tissues and, in contrast to humans, will usually hemorrhage when incised by a sharp scalpel or scissors. Dorsal and ventral branches from the medial and lateral long posterior ciliary arteries and veins enter the basal iris or anterior ciliary body to form an incomplete vascular circle providing the majority of the blood supply to the anterior uvea (Fig. 9.7). In dogs, this incomplete vascular annular circle occupies the basal iris in about 50% of animals; in the remaining animals the vascular circle is positioned in the anterior portion of the ciliary body. As a result, hemorrhage occurs about one-half of the time when the basal iris is incised. Radial arteriolar branches from this circle terminate in capillary beds in the animal pupil. The minor arteriolar circle of the iris, observed in humans, is lacking or incomplete in many animal species. Incision of the basal iris of animals is expected to hemorrhage and may require cautery for hemostasis, which, if possible, should be performed with the peripheral iris protracted from the anterior chamber. The inflamed animal iris can markedly thicken; this increased thickness is a significant deterrent to surgical- and laser-produced iridotomies, and unfortunately these small holes will usually seal and close within a few days.
The animal iris is remarkably similar microscopically among the mammalian animal species, with often the main difference being the size and shape of the pupil. The iris separates the anterior and posterior chamber, with the former considerably larger. Aqueous humor, produced primarily by ciliary body processes, flows from the posterior chamber, through the pupil to enter the anterior chamber, and eventually exits the conventional and uveoscleral outflow passages. The different layers of the iris (from anterior to posterior) include: 1) the anterior border; 2) stroma and iridal sphincter musculature; and 3) the posterior epithelial layers including the iridal dilator muscles (Fig. 9.8). The anterior border of the iris, consisting of fibroblasts and melanocytes, has direct contact with the aqueous humor, and forms a more-or-less continuous cellular surface. The iris stroma consists of fine collagenous fibers, fibroblasts and melanocytes, and numerous blood vessels. The considerable extracellular spaces accommodate the physical changes in iris size secondary to the variations in pupil size.
Ciliary body
The ciliary body is the second component of the anterior uvea, and continues posteriorly with the choroid. The ciliary body has contact with both anterior and posterior chambers, the sclera externally, the lens and vitreous internally, and the retina and choroid posteriorly (Fig. 9.9). The ciliary body is the principal source of aqueous humor, controls accommodation, and is important in the control of intraocular pressure (IOP). Aqueous humor is produced by active secretion by the non-pigmented ciliary body epithelium and by ultrafiltration from the capillaries in the ciliary body processes. Aqueous humor provides nutrients and removes waste products for the lens, anterior vitreous, iris, and posterior cornea. Aqueous humor, once leaving the ciliary body processes, enters the posterior chamber, traverses the pupil, and exits the anterior chamber through the iridocorneal angle and trabecular meshwork including the cilioscleral cleft or sinus within the anterior aspect of the ciliary body, or posteriorly through the uveoscleral route. Hence, the aqueous humor dynamics of the ciliary body are directly related to maintenance of IOP, essential for most of the intraocular tissues’ health and functions. The ciliary body, through its musculature, changes the tension on the zonulary attachments to the lens equator, and effects accommodation. Accommodation, or changes in the anterior–posterior length of the lens, appears minor in most mammals, and is seen mainly in young animals. Accommodation and the ciliary body musculature in non-human primates, some avian species, and humans are highly developed.
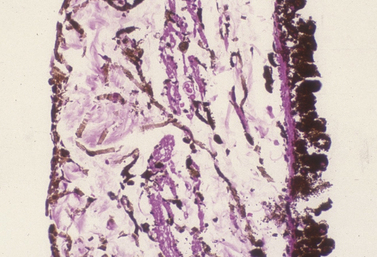
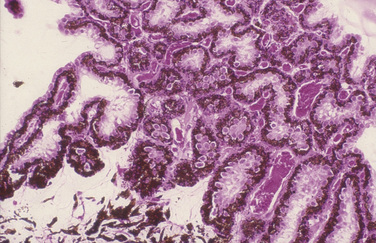
Microscopically the ciliary processes consist of a core of stroma rich in blood vessels, and two layers of epithelium (Fig. 9.10). The inner non-pigmented ciliary epithelium’s primary function is the formation of aqueous humor. It continues posteriorly as the inner neurosensory retina. The non-pigmented ciliary epithelia are linked to each other with tight gap junctions that represent ultrastructurally the blood–aqueous barrier. The deeper pigmented ciliary epithelium provides the majority of the pigmentation of the ciliary processes. The core of connective tissue and blood vessels within the ciliary process supply the energy needs of the two layers of epithelia, and the ultrafiltration portion of the aqueous humor. The more external aspect of the ciliary body consists of smooth muscles. With limited accommodation, the primary ciliary body musculature in the cat and dog is meridionally arranged and extends to the iridocorneal angle, forming the collagen beams for the trabecular meshwork. These ciliary muscles are richly innervated with parasympathetic nerve endings. Ciliary blood vessels are surrounded with numerous sympathetic nerve endings.
Pathophysiology
Pre- and postoperative treatment with topical and systemic corticosteroids and NSAIDs, and intracameral heparin during surgery help to reduce the formation of intraocular fibrin. Introduction of tissue plasminogen activator (tPA), injected directly into the anterior chamber, can dissolve any existing aqueous humor fibrin and clotted blood within 1–2 weeks post-formation. Intracameral tPA cannot, however, prevent the formation of future aqueous humor fibrin. With any postoperative iridocyclitis, the iris becomes inflamed and thicker than normal. From its inflammatory products the iridal surface becomes sticky and readily adheres to any intraocular tissues it contacts. As a result, temporary to permanent attachments may develop involving the iris and lens (posterior synechiae), the iridocorneal angle (peripheral anterior synechiae), the posterior cornea (anterior synechiae), or its pupillary margins (pupillary synechiae). Permanent iridal contact with the anterior lens capsule results in anterior capsular and anterior cortical cataract formation. Permanent iridal contact with the posterior cornea results in edema and dense corneal scars. Formation of peripheral anterior synechiae within the iridocorneal angle predisposes the eye to angle-closure glaucoma by filling and closing the opening of the sclerociliary cleft. Adherence of the pupillary margins creates an irregular pupil and may completely occlude the pupil, resulting in an immediate iris bombé and loss of vision. Treatment strategies to prevent the formation of synechiae are summarized in Box 9.1.
Box 9.1 Benefits of iridocycloplegia during iridocyclitis
• Administration of a mydriatic or combination of mydriatics to constantly change the pupil size, produce iridal movement, and discourage iridal attachments to the lens or posterior cornea.
• Dilatation of the pupil to position the majority of the iris near the peripheral lens and away from the closer central and visually important axis.
• Dilatation of the pupil to prevent obstruction with inflammatory materials or formation of annular (360°) posterior synechiae.
• Suppress iridociliary inflammation and tissue swelling.
• Paralyze the iridal sphincter and ciliary body musculature to minimize the pain from iridocyclitis.
• Restore the blood–aqueous barrier to decrease as much as possible the cellular and protein (fibrin) content of the secondary or plasmoid aqueous humor, and reduce the possibility of formation of fibropupillary membranes.
Surgery of the anterior chamber
Keratocentesis/anterior chamber paracentesis
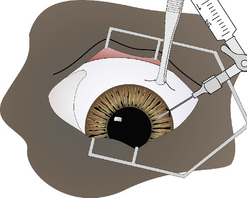
In keratocentesis a small gauge (25–30) hypodermic needle is inserted into the peripheral clear cornea or limbus to enter the anterior chamber and aspirate a small amount (0.1–0.2 mL) of aqueous humor. Alternatively, this technique may also be used for intracameral injection of materials such as tPA, adrenaline (epinephrine) or antibiotics. The indications for keratocentesis are summarized in Box 9.2. The aqueous humor sample has a limited volume, usually 0.1–0.3 mL. The value of each diagnostic procedure (cytology, culture, protein analyses, antibody titers) may require prioritization and only the most important tests performed (Fig. 9.11).
Box 9.2 Indications for keratocentesis in animals
• Aqueous humor cytology may assist in the diagnosis of the anterior uveal inflammation.
• Aqueous humor culture may determine the infectious organism.
• Aqueous humor titers to selected diseases.
• Aspiration of small iris/ciliary body cysts.
• To abruptly decrease intraocular pressure in patients with medically non-responsive glaucoma.
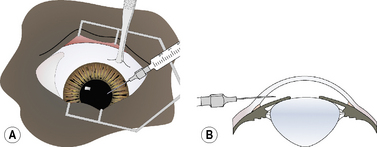
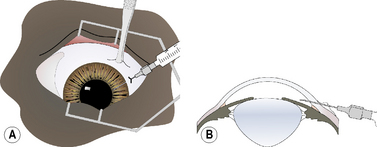
For keratocentesis, a 25–30 g hypodermic needle and 1 mL syringe are used (Fig. 9.12a). A thumb forceps is used to grasp the bulbar conjunctiva and stabilize the eye. The hypodermic needle is directed through the peripheral cornea into the anterior chamber at an angle that avoids contact with the posterior cornea and the anterior iris (Fig. 9.12b). A small volume (0.1–0.3 mL) of aqueous humor is withdrawn. The needle bevel may be positioned down (or toward the iris) to avoiding snagging the iris and to provide a self-sealing needle puncture.
As a variation of the clear corneal method, the hypodermic needle is inserted in the bulbar conjunctiva 2–4 mm posterior to the limbus (Fig. 9.13a). It is then carefully moved forward subconjunctivally to the limbus, and then inserted through the limbus at an angle between the posterior cornea and anterior iris (Fig. 9.13b). After aqueous humor sampling, the hypodermic needle is carefully and slowly retracted. If some aqueous humor leaks from the limbal penetration, it remains trapped in the bulbar subconjunctival space.
An alternative keratocentesis technique uses two 1 mL syringes connected by a two-way stopcock, and a single 25–30 g hypodermic needle. One syringe is used to aspirate 0.1–0.3 mL of aqueous humor; the other is filled with 0.5 mL sterile lactated Ringer’s or balanced salt solution (BSS), and is used to refill the anterior chamber with a volume equal to the aqueous humor removed (Fig. 9.14). This technique immediately replaces the lost aqueous humor, reduces the influx of the plasmoid or secondary aqueous humor, avoids any ocular hypotony, and requires only a single needle penetration into the anterior chamber.
Corneal/limbal incisions
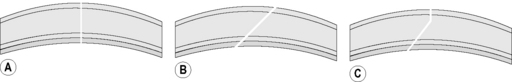
The peripheral cornea and limbus may be incised perpendicular to the corneal surface, as a beveled incision to the corneal surface, or an incision that combines both perpendicular and beveled characteristics (Fig. 9.15). Those entries that include a beveled or angled entry into the anterior chamber are self-sealing. Entry into the anterior chamber that involves incisions that are perpendicular to the corneal surface are not self-sealing, but result in the least corneal scar formation. Limbal incisions are usually performed under a limbal- or fornix- based bulbar conjunctival flap. If a limbal-based conjunctival flap is used, the flap is usually only 3–5 mm wide to minimize its adverse effect on observation of the anterior chamber during iris and lens surgeries. Sutures for closure of the limbal wound may be buried under the limbal- or fornix- based conjunctival flap, or the knots may be exposed at the external limbus. Suturing of the limbal wound under a limbal-based conjunctival flap is more tedious as braided sutures often snag on the flap.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree