Chapter 10 Surgical procedures for the glaucomas
Introduction
The canine glaucomas
Glaucomas in small animals may be divided by gonioscopy (examination of the iridocorneal anterior chamber angle) and etiology into primary, secondary, and congenital (Box 10.1). Primary glaucomas affect both eyes, although one eye may demonstrate clinical signs of glaucoma several months to years before the opposite eye. Traditionally, the symptoms of primary glaucomas are divided into acute and chronic. However, clinical experience suggests that many of the primary breed-related glaucomas are chronic in their development, with acute elevations in IOP toward the end of the disease that produce overt clinical signs which prompt presentation to the veterinarian. This suggests that treatment for most primary glaucomas in dogs is started with the disease in an already quite advanced state.
Box 10.1 The different types of glaucoma in animals
Secondary: open/narrow/closed angle – all species
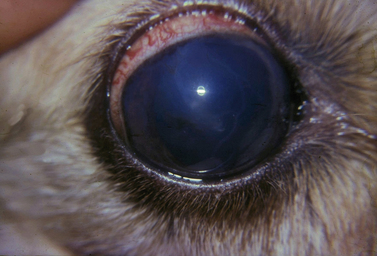
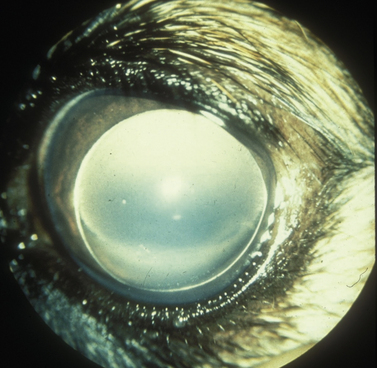
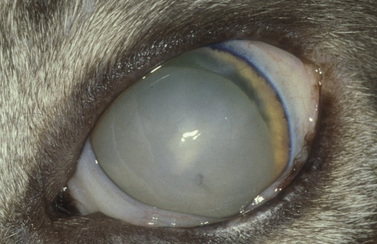
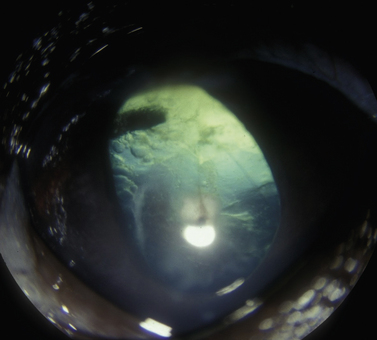
The clinical signs of acute and often marked elevated levels of IOP, associated with primary closed-angle glaucoma, are a dilated, fixed, or sluggish pupil, bulbar conjunctival and episcleral venous congestion, and corneal edema, as well as patient discomfort and visual disturbances (Fig. 10.1). With prolonged elevations of IOP, secondary enlargement of the globe, lens displacement, breaks in Descemet’s membrane of the cornea, and eventual buphthalmos (enlargement of the globe due to stretching) result (Fig. 10.2). Pain is usually manifested by behavioral changes and sometimes periorbital pain rather than blepharospasm, or not at all.
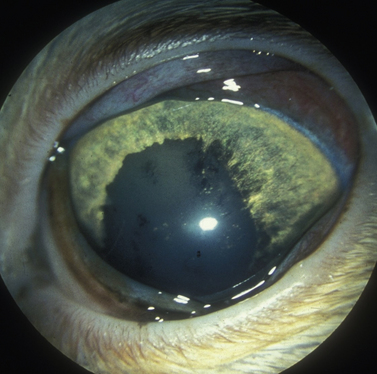
Treatment of the secondary glaucomas, which are often unilateral, is determined based on the underlying disease. The most frequent cause of secondary glaucoma in the dog is lens displacement, manifested as subluxation, anterior luxation, or posterior luxation (Fig. 10.3). However, with megaloglobus and subtle but gradual increases in IOP, secondary lens subluxation is also common in the primary glaucomas as tension of the lens zonules eventually results in their tearing, usually just below their attachment to the lens equator. In addition, lens-induced uveitis (LIU) is common in the dog, secondary to leakage of lens proteins from cataracts. Occasionally the iridocorneal angles of globes affected by LIU become plugged with lens proteins and inflammatory cells; peripheral anterior synechiae then form, sufficient to elevate IOP. In both of these mechanisms of secondary glaucoma in dogs, lens or cataract removal may be important in the treatment of elevated IOP.
New glaucomas, observed in dogs, are those occurring secondary to silicone oil in the anterior chamber, and those secondary to rhegmatogenous retinal detachments. Silicone oil is used in the repair of retinal detachments in dogs (see Chapter 12). Leakage of this oil into the anterior chamber results in epithelial toxicity, impaired aqueous outflow, and increased IOP, and the oil must be removed from the anterior chamber. Subconjunctival silicone oil appears relatively inert.
The feline glaucomas
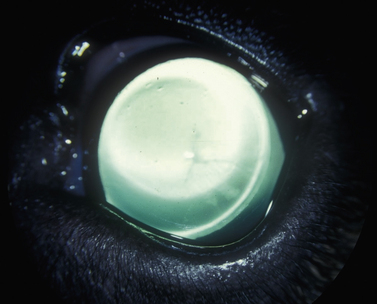
Glaucomas in cats occur predominately secondary to anterior uveitis and neoplasia; however, primary open-angle glaucoma also occurs at a very low frequency. In one report, based on 131 enucleated eyes, feline glaucomas were associated with chronic lymphocytic–plasmacytic anterior uveitis (53 eyes), diffuse iridal melanoma/melanocytoma (38 eyes), other neoplasms (14 eyes), lens rupture (4 eyes), anterior lens luxation (4 eyes), primary glaucoma (3 eyes), and other causes (15 eyes). In clinical reports, the most frequent form of glaucoma in cats occurs secondary to anterior uveitis, and neoplasia (Fig. 10.4). In cats with inflammatory-related secondary glaucomas, topical and systemic corticosteroids frequently reduce the anterior uveitis sufficiently to lower IOP. Cats, usually older than 10 years, may also develop lens luxation in the absence of iridocyclitis (Fig. 10.5). Some feline eyes have normal levels of IOP; in others the IOP is elevated. The globe is usually slightly enlarged, but the eye often remains visual. Gonioscopy of affected cats (which, unlike dogs, may be performed without the aid of a refracting goniolens) usually reveals open iridocorneal angles; in fact, some iridocorneal angles appear recessed posteriorly in enlarged globes and are wider than normal. Lens removal in normotensive eyes may resolve the problem. If glaucoma is already present, lens removal alone may not sufficiently address the elevated IOP because of permanent outflow abnormalities.
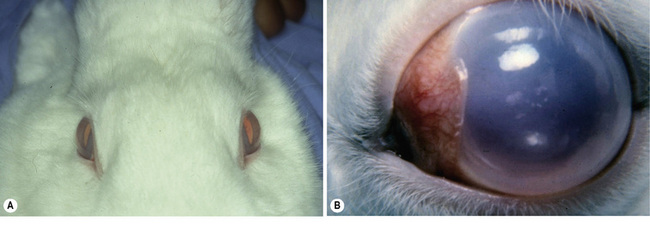
Congenital glaucoma in rabbits
In affected rabbits, impaired aqueous humor outflow occurs by 3 months, and IOP elevates by 6 months. Corneal and globe enlargement are detectable in animals as young as 4–6 months old. Pet rabbits are typically presented with, at least, one buphthalmic eye (Fig. 10.6). Corneal edema, pupillary dilatation, and elevated IOP are present. Medical treatment of pet rabbits is generally limited to topical agents; however, miotics and beta adrenergics are less effective and of shorter duration than in dogs and cats. Glaucoma surgical procedures, including iridencleisis, can provide normal IOP for several months. Anterior chamber shunts in congenital glaucomatous rabbits have been successful for periods greater than 3 years; however, rabbits generally exhibit an even more aggressive healing and subsequent scarring response at the site of surgical intervention than dogs and cats.
Diagnostic and monitoring techniques for glaucoma patients
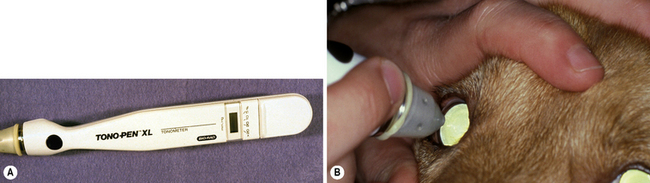
Only applanation tonometers are used in all of the different animal species to estimate IOP, and fortunately are fairly accurate (in spite of varying corneal diameters) within the normal range of IOP, though they tend to underestimate when pressure exceeds about 40 mmHg (Fig. 10.7). Rebound tonometry is increasingly used to estimate IOP based on its ease and excellent patient compliance. Ophthalmoscopy, especially the direct method, permits detection of IOP-related damage to the retina and optic disk.
Gonioscopy, or the direct observation of the iridocorneal angle through a special contact goniolens, is the basis for classification of all glaucomas, monitors iridocorneal angle and cleft changes as the glaucoma progresses, and assists selection of the different medical and surgical treatments (Figs 10.8 and 10.9). In most primary open-angle and narrow-angle glaucomas in animals, the iridocorneal angle, as viewed by gonioscopy, progressively narrows and eventually closes with the secondary formation of peripheral anterior synechiae.
Glaucoma classification as a guide to therapy
Classification of the etiology of glaucoma, based on ophthalmic, gonioscopic, and ultrasonographic examinations, assists in the optimal planning of clinical management and the preservation of vision (Box 10.2). With progressive iridocorneal angle closure that occurs in most canine glaucomas, the choice of medical, surgical or, most frequently a combination of both modalities, must be tailored. In the case of acute goniodysgenic/angle-closure glaucoma in dogs, surgical treatment is the first choice, with medical treatment usually providing only a few weeks to months of effective IOP control. Unfortunately, surgical treatments for the primary canine glaucomas and the maintenance of effective long-term IOP control have been notoriously challenging. However, diode laser cyclophotocoagulation and anterior chamber shunts appear to offer longer periods of successful control of IOP at this time. In horses, laser cyclophotocoagulation appears more successful than in dogs, although use of anterior chamber shunts is still under study.
Box 10.2 Recommended medical and surgical treatment modalities for the different glaucomas in animals
Primary open angle glaucoma
Short and long term
Surgical anatomy
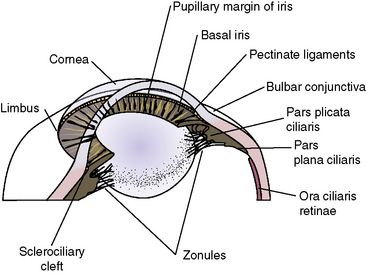
Procedures for the surgical management of glaucoma require accurate knowledge of the anterior orbit, globe, and intraocular tissues, including the iridocorneal angle, lens, iris, and ciliary body (Fig. 10.10). The anatomy of the last three structures (the iris and ciliary body, and the lens) is presented in Chapters 9 and 11, respectively. However, some additional comments regarding the anatomy of this area are important for the effective performance of the different glaucoma surgical procedures.
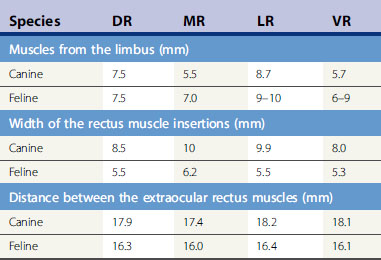
When cyclodialysis, posterior sclerotomy, and anterior chamber shunts are performed in small animals, the surgeon should be familiar with the anterior orbit and, in particular, the extraocular muscular insertions to the globe (Table 10.1). The dog orbit has considerably more room for instrument manipulations and glaucoma surgeries than the cat. Entry through the sclera (posterior sclerotomy for cyclodialysis) is usually performed at the 12 o’clock position because of improved surgical exposure. Like cataract patients, use of neuromuscular blocking drugs during surgery enhances maximal exposure of the globe, reduces intraorbital pressure on the globe, and facilitates anterior chamber surgeries. Placement of the episcleral portion of the anterior chamber shunts is usually performed between the dorsal rectus muscle and medial or lateral rectus muscle insertions. If the anterior chamber shunt is wider than the space between these muscles, portions of it may be manipulated under these muscles. If the implant is long, it may extend posteriorly beyond the equator of the globe and upon the insertions of the retractor oculi muscles. Subsequent implants (if deemed clinically necessary) may be placed in the dorsonasal, ventrolateral, and ventronasal quadrants in that order of preference. At the insertions of the major rectus muscles, both arterial and venous connections with the anterior globe occur. Incision of these rectus muscle insertions should be avoided since any hemorrhage in the wound field will further disseminate and stimulate those growth factors which mediate subsequent bleb fibrosis and failure. Meticulous wet-field cautery facilitates a blood-free surgical site. With most gonioimplants, placement at least 10–14 mm behind the limbus is recommended in dogs.
Table 10.1 Anatomy of the rectus muscles in dogs and cats: Mean distance of the extraocular insertions of the dorsal (DR), medial (MR), lateral (LR), and ventral (VR), and rectus
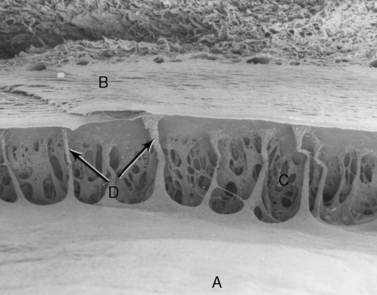
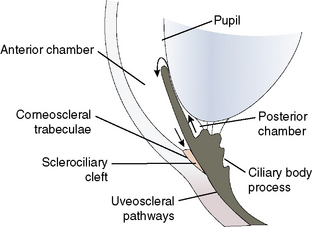
While the macroscopic anatomy of the iridocorneal or anterior chamber angle of the dog and cat differs somewhat from the radial canal of Schlemm in humans and non-human primates, the ultrastructure of the trabecular meshwork and the aqueous humor dynamics are remarkably similar (Fig. 10.11). In dogs and humans the percentage of aqueous humor that exits the trabecular meshwork is about 85–90% (conventional outflow), and about 10–15% leaves by the uveoscleral route. The rates of aqueous humor turnover in dogs, cats, and humans appear quite similar (about 5.0–6.0 μL/min), although cats have been thought to have a much higher rate (about 15 μL/min). In horses, the best studies to date suggest that the majority of aqueous humor leaves through the uveoscleral outflow pathway. Hence, aqueous humor gradually flows posteriorly through the base of the iris and ciliary body to enter the subscleral space between the choroid and sclera.
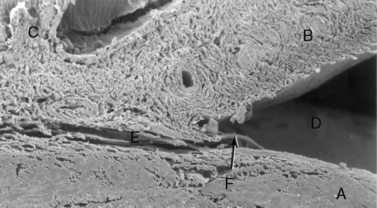
Aqueous humor outflow pathways have the following boundaries: 1) the limbus and anterior chamber; 2) inwardly the pectinate ligaments, base of the iris, and inner aspects of the ciliary or sclerociliary cleft or sinus; 3) posteriorly the deeper aspects of the ciliary cleft or sinus; and 4) outwardly the sclera anteriorly and the outer aspects of the ciliary or sclerociliary cleft posteriorly (Fig. 10.12). Canine and feline aqueous outflow pathways differ slightly from those of humans by a cleavage of the anterior ciliary body which contains most of the trabeculae, and the iris base that directly communicates with the peripheral anterior chamber. Pectinate ligaments of various sizes and shapes connect the inner posterior limbus and termination of Descemet’s membrane to the anterior base of the iris. Although these pectinate ligaments appear to play no direct role in aqueous humor outflow unless significantly malformed/imperforate, they do provide stability for the iris. These iridocorneal angle anatomic differences between humans, non-human primates, and other animal species are thought to be associated with lens accommodation rather any differences in aqueous humor outflow physiology.
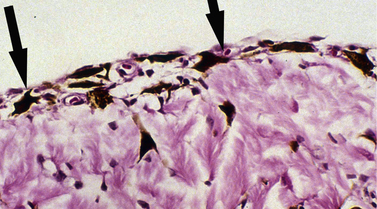
Pre-iridal fibrovascular membranes (PIFMs) are common in advanced glaucomas in dogs. These membranes are potential sources of fibrin and other proteins, as well as erythrocytes and inflammatory cells that are released when the anterior chamber is opened and IOP lowered abruptly to 0 mmHg (Fig. 10.13). These same membranes may bridge the anterior iridocorneal angle, contributing to the formation of peripheral anterior synechiae (PAS), and even extend across the pupil and anterior lens capsule, and into the posterior chamber.
Potentially the most significant complication associated with the glaucoma surgeries is the pronounced inflammatory response which occurs at the surgical site in which aqueous humor is redirected into the episcleral space and surrounding tissues. This response results in considerable fibrosis about the area, and ultimately contributes to the failure of filtration blebs or drainage setons. Of interest, the normal passage of aqueous humor through the uveoscleral pathway all the way posterior to the optic nerve head does not incite this inflammatory response! Although not fully understood, the mechanism of this response appears to follow the general parameters of the wound-healing cascade, with growth factors mediating fibroblastic recruitment and transformation. Connective tissue growth factor (CTGF) and transforming growth factor beta 2 (TGF-β2) have been shown to mediate these initial events in multiple animal models (Tables 10.2a and 10.2b). Subsequent tissue fibrosis and cicatricial remodeling is at least partially matrix metalloproteinase mediated. Successfully controlling this postoperative fibrotic response is crucial to improving the long-term success rates of all glaucoma surgeries. Ultimately, this may require a ‘chemotherapeutic’ approach involving multiple targeted intraoperative and postoperative medications (applied topically as well as systemically). Achieving this goal represents the single greatest challenge in the successful long-term surgical treatment of this disease complex!
Table 10.2a Matrix metalloproteinases (MMPs) important in ocular wound healing
| MMP common name | Designation | Substrates and actions |
|---|---|---|
| Fibroblast collagenase | (MMP-1) | Cleaves single bond in native types I, II and III collagens |
| 72 kDa Gelatinase | (MMP-2) | Degrades types IV, V, and VII collagens, gelatin, fibronectin; synthesized by fibroblasts and macrophages |
| 92 kDa Gelatinase | (MMP-9) | Degrades types IV and V collagen, gelatin; synthesized by epithelial cells, macrophages, polymorphonuclear leukocytes |
| Stromelysin | (MMP-3) | Degrades proteoglycans, fibronectin, laminin, gelatin and types III, IV, and V collagen |
| Neutrophil collagenase | (MMP-8) | Similar to MMP-1, degrades type I, II, and III collagen |
Table 10.2b Growth factors and cytokines important in ocular wound healing
| Abbreviation | Description | |
|---|---|---|
| Growth factor | ||
| Epidermal growth factor | EGF | Synthesized by corneal epithelial cells, lacrimal gland; mitogen and chemotactic factor for all three types of corneal cell |
| Transforming growth factor alpha | TGF-α | Structurally and functionally similar to EGF; synthesized by corneal epithelial cells, lacrimal gland |
| Transforming growth factor beta | TGF-β | Three isoforms: TGF-β1, TGF-β2, TGF-β3; promotes formation of extracellular matrix; TGF-β2 present in aqueous humor; synthesized by multiple types of cell |
| Basic fibroblast growth factor | bFGF | Detected in basement membranes; synthesized by endothelial cells; mitogen for fibroblasts and endothelial cells; angiogenic |
| Acidic fibroblast growth factor | aFGF | Detected in basal layer of corneal epithelial cells and basement membranes; synthesized by endothelial cells; mitogen for fibroblasts and endothelial cells; angiogenic |
| Keratinocyte growth factor | KGF | Synthesized by keratocytes; stimulates corneal epidermal cell proliferation and migration |
| Hepatocyte growth factor | HGF | Synthesized by corneal epithelial cells; stimulates corneal epidermal cell proliferation and migration |
| Platelet-derived growth factor | PDGF | Synthesized by corneal epithelial cells; stimulates proliferation of stromal fibroblasts |
| Insulin-like growth factor I | IGF-I | Part of the IGF axis (comprising surface receptors, ligands, binding proteins and proteases). Involved in early healing and promotes cellular proliferation. |
| Connective tissue growth factor | CTGF | Stimulates fibrosis and mediates the actions of TGF-β on matrix formation |
| Cytokines | ||
| Tumor necrosis factor alpha | TNF-α | Proinflammatory cytokine with multiple effects including chemotaxis of leukocytes, increased production of MMPs, and induction of apoptosis; soluble TNF-α is released by TACE cleavage of transmembrane pro-TNF-α |
| Interleukin-1 beta | IL-1β | Proinflammatory cytokine synthesized by corneal epithelial cells; stimulates MMP production by keratocytes |
TACE, tumor necrosis factor-α converting enzyme.
Preoperative treatment
Surgical entry into the anterior chamber when IOP is 25 mmHg or higher can be hazardous to the eye and surgical outcome. If necessary, additional vigorous massage and hypotensive medical treatment should be initiated (such as an additional intravenous dose of osmotic agents) before the eye is entered. Glaucoma procedures in a globe with elevated IOP exhibit higher risk and lower surgical success rates. A glaucomatous eye without satisfactory ocular hypotension upon entry may exhibit choroidal hemorrhage, choroidal edema, vitreous protrusion through the pupil, and forward displacement of the iris. Intraocular hemorrhage is also more apt to occur in these globes. Cautious anterior chamber paracentesis may be performed in a glaucomatous eye that has not responded to vigorous medical treatment. Keratocentesis is usually performed under general anesthesia or deep sedation, and aqueous humor is removed slowly from the anterior chamber after first decompressing the syringe plunger (see Chapter 9).
Surgical procedures for the glaucomas
Surgical procedures for the treatment of the glaucomas may be divided into two types: 1) those that increase outflow of aqueous humor via alternative pathways of drainage; and 2) those that decrease the rate of formation of aqueous humor by destroying part of the pars plicata or corona ciliaris (Table 10.3). Procedures to increase the outflow of aqueous humor include iridencleisis, corneoscleral trephination, cyclodialysis, combined iridencleisis and cyclodialysis, posterior sclerectomy, and anterior chamber shunts (gonioimplants). Additional procedures to increase the outflow of aqueous humor include goniopuncture, iridosclerectomy, goniotomy, trabeculectomy, and sinusotomy, but these surgical procedures have not been described in dogs. Techniques used to reduce the rate of aqueous humor formation by partial destruction of the ciliary body include cyclocryothermy, cyclodiathermy, and diode laser transscleral or endoscopic cyclophotocoagulation. The popularity, staging, and combining of these different glaucoma surgical techniques are constantly evolving. However, as new antifibrosis drugs are developed, some of these older traditional selected filtration procedures may again be more useful.
Table 10.3 Mechanisms of surgical treatments for the glaucomas
| Mechanism | Type of surgery |
|---|---|
| Filtration | Iridencleisis |
| Filtration/decrease formation* | Cyclodialysis |
| Pupil bypass | Iridectomy |
| Filtration/decrease formation* | Iridencleisis/cyclodialysis |
| Iridocorneal angle bypass | Corneoscleral trephination |
| Filtration/decrease formation | Cyclodialysis/iridocyclectomy |
| Iridocorneal angle bypass | Anterior chamber shunts/gonioimplants |
| Decrease aqueous formation | Cyclocryotherapy |
| Decrease aqueous formation | Transscleral or endoscopic cyclophotocoagulation (diode laser) |
| Destroy ciliary body epithelia | Intravitreal gentamicin |
Iridencleisis
In the iridencleisis procedure, a radial section of iris is permanently positioned through a limbal incision into the subconjunctival spaces beneath the bulbar conjunctiva (Fig. 10.14). Reduction in IOP following iridencleisis is primarily related to the escape of aqueous humor through this area. Aqueous may also filter through the space between the two pillars of the iris, as well as through the iridal stroma itself. Iridencleisis may be used successfully in the management of narrow- and closed-angle glaucoma, acute iris bombé associated with annular posterior synechiae, and glaucoma associated with peripheral anterior synechiae. When the iris is thin, atrophied, or adhered to the lens with focal posterior synechiae, iridencleisis is not recommended.
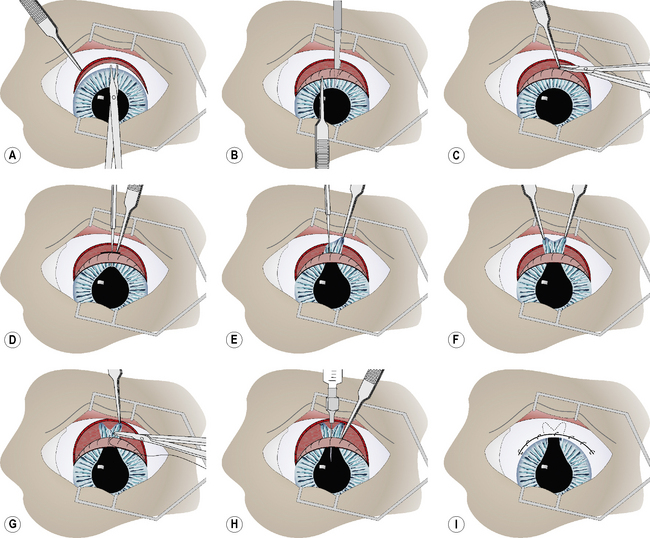
With curved blunt-tipped tenotomy scissors, a limbal-based 10 mm bulbar conjunctival flap is constructed. The flap is usually 12–18 mm long. Tenon’s capsule is identified and, if extensive, excised from the overlying bulbar conjunctiva and sclera in the area of the limbal incision (Fig. 10.15a). The anterior chamber is entered through the limbus with the Beaver No. 6500 microsurgical (Fig. 10.15b). The limbal incision is usually 8–10 mm long. A 1–2 mm section of sclera in the caudal aspect of the limbal incision is carefully excised (anterior sclerectomy). Hemostasis and limited tissue destruction are achieved by cautious electrocautery (Fig. 10.15c). The wet-field coagulator is superior in this region because of the frequent presence of aqueous humor. Limited application of electrocautery on the scleral aspect of the incision seems to facilitate maintenance of an open fistula and reduces the possibility of closure by fibrosis.
During the limbal incision and subsequent anterior sclerectomy, the iris may protrude into the incision. A blunt iris hook or serrated iris forceps is carefully manipulated into the anterior chamber to grasp the dorsal pupillary margin and protract the iris into the limbal incision (Fig. 10.15d). Using the iris hook and serrated forceps, the iris is carefully pulled from the anterior chamber into the limbal incision (Fig. 10.15e). With both iris forceps pulling in opposite directions, the iris is slowly torn radially to its base. Each pillar of the iris, with its pigmented epithelium exposed, is manipulated into the respective end of the limbal incision (Fig. 10.15f). To minimize the possibility of the iris pillar retracting back into the anterior chamber, each tag of the iris is attached to the sclera with a 6-0 simple interrupted absorbable suture (Fig. 10.15g). The anterior chamber is carefully irrigated with balanced salt solution to remove all fibrin and blood. With successful manipulation of the iris and judicious use of electrocautery, hemorrhage and fibrin in the anterior chamber at the conclusion of surgery are generally avoided. In the event that fibrin remains, or continues to be formed in the anterior chamber, tissue plasminogen activator (tPA; 25–50 μg) is injected between the two iris pillars into the anterior chamber (Fig. 10.15h).
The limbal wound is not apposed with sutures. The bulbar conjunctival flap is apposed with a 6-0 to 7-0 simple continuous absorbable suture (Fig. 10.15i).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree