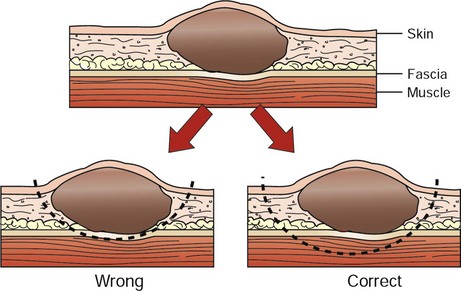
10 Complete surgical removal of localized cancer cures more cancer patients than any other form of treatment,1 in part because this modality is generally applied as sole treatment for local disease, early stage disease, or tumors with limited potential to metastasize. In humans, 60% of patients who are cured of cancer are cured by surgery alone.2 Before this hope for cure can be realized in veterinary medicine, surgeons must have a thorough understanding of anatomy, physiology, resection, and reconstruction options for all organs; expected tumor behavior; and the various alternatives or adjuvants to surgery. Surgical oncologists should not only be good technical surgeons, but also dedicated tumor biologists. Surgery will likely play a role at one point or another in the management of most cancer patients. Surgical procedures may include any of the following: diagnosis (biopsy), resection for cure, palliation of symptoms, debulking (tumor cell cytoreduction), and a wide variety of ancillary procedures to enhance and complement other forms of treatment. Most patients with cancer are “old.” However, “old” is a relative term, and a geriatric dog or cat with normal organ function should not be denied treatment simply on the basis of age. Age has not been shown to impact tumor-related prognosis. In fact, dogs with osteosarcoma that are less than 2 years of age do worse than dogs that are more than 2 years of age after amputation alone.3 “Old” animals, in most instances, will tolerate aggressive surgical intervention as well or as poorly as “young” patients. Although biopsy principles are covered in Chapter 9, it bears emphasizing that properly timed, performed, and interpreted biopsies are one of the most crucial steps in the management of the cancer patient. Not only does the surgeon need to procure adequate and representative tissue to establish a diagnosis, but also the biopsy must not compromise subsequent curative surgical resection or radiation field planning. 1. What is the histologic type, stage, and grade of cancer to be treated? 2. What are the expected local and systemic effects of this tumor type, grade, and stage? 3. Is a cure possible and at what price in terms of cosmetics and function? 4. Is an operation indicated at all? 5. What are the options for alternative or planned combination treatment? The surgical oncologist must be able to assimilate all of the information and make an informed decision. We must also remind ourselves and our clients that there is much we do not know (e.g., incomplete margins do not necessarily ensure later local recurrence4) and that surgical judgment regarding expected local behavior and likely resection is often qualitative and is an imperfect “science.” 1. All incisional biopsy tracts should be excised in continuity with the primary tumor because tumor cells are capable of growth in these wounds. Fine-needle aspiration (FNA) cytology tracts are of minor, but not zero, concern, whereas punch biopsy tracts are of intermediate concern.5 With this in mind, all biopsies should be positioned in such a manner that they can be removed at surgery. 2. Early vascular ligation (especially venous) should be attempted to diminish release of large tumor emboli into the systemic circulation. This is probably only clinically meaningful for those tumors with a well-defined venous supply, such as splenic tumors, retained testicles, and lung tumors. Small numbers of cancer cells are constantly being released into the venous (and lymphatic) circulation by most tumors. Larger, macroscopic cell aggregates may be more dangerous, however, and these may be prevented from vascular escape with early venous ligation. 3. Local control of malignant cancer requires that a margin of normal tissue be removed around the tumor. Resection of the “bad from the good” can and should be classified in more detail than radical versus conservative (Table 10-1).6 Tumors with high probability of local recurrence (e.g., high-grade soft tissue sarcoma, high-grade mast cell tumors, feline mammary adenocarcinoma) should have 2- to 3-cm margins removed in three dimensions. Tumors are not flat, and wide removal in one plane does not ensure complete excision. Fixation of cancer to adjacent structures mandates removal of the adherent area in continuity with the tumor. This is commonly seen with oral cancer that is firmly adherent to the underlying mandible or maxilla. Invasive cancer should not be peeled out, shelled out, enucleated, or curetted if a cure is expected. Many cancers are surrounded by a pseudocapsule. This capsule is almost invariably composed of compressed and viable tumor cells, not healthy reactive host cells. If a malignant tumor is entered at the time of resection, or if the margins are incomplete, that procedure is often no better therapeutically than a large incisional biopsy. When possible, resection of the previous scar and the entire wound bed with “new” margins (never entering the old wound cavity) is indicated. One should strive for a level of dissection that is one tissue plane away from the mass (Figure 10-1). For example, invasion of cancer into the medullary cavity of a bone requires subtotal or total bone resection and not curettage. Table 10-1 Classification and Resection of Wound Margins 4. Tumors should be handled gently to avoid risk of breaking off tumor cells into the operative wound, where they may thrive.7 Copious lavage of all cancer wound beds will help mechanically remove small numbers of exfoliated tumor cells but should not replace gentle tissue handling and avoidance of entering the tumor bed. 5. If more than one malignant mass is being removed, separate surgical packs should be used for each site to avoid iatrogenic tumor cell implantation from site to site. The aggressiveness of resection should only rarely be tempered by fears of wound closure. It is better to leave a wound partially or even in some cases completely open with no cancer than closed with residual cancer. Numerous innovative reconstructive techniques are available for closure of cancer wounds, and the surgeon is only limited by his or her ingenuity.8 Reliable microvascular-free composite transfers of muscle and skin are somewhat hampered due to unique canine skin/muscle anatomy but are being developed.9 Controversy surrounds the surgical management of regional lymph nodes draining the primary tumor site.10,11 As a general rule, epithelial cancers are more likely to metastasize to lymph nodes than are mesenchymal cancers. However, any enlarged regional lymph node requires investigation for complete staging. Lymphadenopathy may be from metastasis of cancer (firm, irregular, and sometimes fixed to surrounding tissue) or from hyperplasia and reactivity to various tumor factors, infection, or inflammation.12 The former cause is a poor prognostic sign, and the latter may be a beneficial host response. Enlarged lymph nodes as a result of cancer metastasis and invasion are generally effaced by tumor cells and can often be diagnosed by FNA. Histologically, positive lymph nodes at diagnosis usually are a sign of impending emergence of systemic metastasis. Removal of lymph nodes should be considered under the following circumstances: 1. If the lymph node is positive for cancer and not fixed to surrounding normal tissues, it may be possible to remove the node with some therapeutic intent. Frequently, however, many lymph nodes drain a primary tumor site (e.g., oral cavity) and lymphadenectomy is incomplete. Lymph node metastasis at the time of initial diagnosis is a poor prognostic sign. However, patients that develop metastasis in a delayed fashion (1 to 2 years) after local tumor control may benefit from lymphadenectomy. Although it is usually not practical, removal of the primary tumor, intervening lymphatic ducts, and draining lymph node has been recommended (en bloc resection). En bloc resection may be possible for a malignant toe tumor with metastasis to the popliteal lymph node but is usually only accomplished with amputation. A mastectomy that includes the regional lymph node (e.g., glands four and five with the inguinal lymph node) is another example of en bloc resection. Few other anatomic sites are routinely amenable to this therapy. A specific instance where local lymphadenectomy may be beneficial is in removal of iliac/sublumbar lymph nodes in patients with metastatic apocrine or sebaceous gland adenocarcinomas of the perineum. Although removal of these lymph nodes is rarely curative, it may help increase the benefit of adjuvant radiation or chemotherapy and should help alleviate, at least in the short term, the paraneoplastic syndrome of hypercalcemia by reducing levels of parathormone-like substances. Regional lymphadenectomy may also prevent or improve obstruction of the large bowel and urinary tract and can be repeated as necessary in the absence of overt systemic metastasis (e.g., metastasis to lungs, liver). 2. It is well estabished that normal-sized lymph nodes may contain micrometastasis, and this point should always be explained clearly to a client. Normal-appearing lymph nodes that are known to drain a primary tumor site may be randomly sampled (biopsy or cytology) to gain further staging information. This is particularly important if adjuvant therapy decisions (irradiation or chemotherapy) would be predicated on confirmation of residual or metastatic cancer. Intrathoracic or intraabdominal lymph nodes are perhaps most crucial because they are not readily accessible to histologic or cytologic follow-up examination. However, in some instances, lymph nodes that are not enlarged cannot be sampled safely due to proximity to vital structures (e.g., sublumbar lymph nodes at the aortic bifurcation), even under ultrasound guidance. In such cases, the surgeon must educate the client about the situation and either remove the primary tumor without further knowledge of regional lymph node involvement or recommend removal of the normal-sized nodes concurrently for staging (and possibly therapeutic) purposes. For dogs with malignant anal sac tumors, this approach involves an exploratory surgery of the sublumbar nodal bed and dissection/removal of all nodal tissue encountered. In human medicine, the concept of the sentinel lymph node (first node to receive lymphatic flow from a primary tumor) has become important in the management of select malignancies, most notably breast cancer.12 Basically, the area of the primary tumor is injected with blue dye or a low dose of a radionuclide or both. The first draining node is detected visually or with a handheld gamma camera probe and removed for frozen section analysis. If the first node is negative for metastasis, subsequent nodal dissection often is avoided. If the sentinel node is positive, further nodal dissection is performed. Targeting the sentinel node is most valuable in an anatomic location in which there is an extensive nodal bed12 and hence numerous potential paths for regional metastasis. The benefits of such an approach in humans are readily apparent because many patients have been spared extensive nodal resections if the sentinel lymph node has been determined to be free of cancer cells. The topic of the sentinel lymph node has only recently emerged in veterinary medicine but is gathering some momentum.13 Several techniques have been described, including microbubble detection via ultrasound,14 fluorescein, and blue dye.15 Although sentinel lymph node staging may not yet have the relevance and importance that have been demonstrated in humans due to less complex nodal networks, there are several potential indications (e.g., tumors of the head and neck) that should be investigated in clinical studies. Histologically positive lymph nodes will alter prognosis and stage and will also be informative for decisions related to postoperative chemotherapy and radiation. Lymph node removal is generally not performed under the following circumstances: 1. Lymph nodes in critical areas (retropharyngeal, hilar, mesenteric) that have eroded through the capsule and become adherent (fixed) to surrounding tissues. In this scenario, lymph nodes may not be removeable without leaving residual disease in the wound bed (necessitating adjuvant therapy to achieve local control) or an attempt at removal may cause serious harm to the patient by injuring important adjacent structures. In such instances, it is usually prudent to aspirate or biopsy the node to confirm involvement in the disease process and leave the node in situ or treat with other modalities. One example of an exception to this scenario is metastasis of limb and paw tumors to prescapular and popliteal lymph nodes that can be removed with amputation (radical en bloc resection). 2. Prophylactic removal of “normal” draining lymph nodes or chains of lymph nodes (as opposed to sampling for stage) is not beneficial and may be harmful.9 Regional lymph nodes may in fact be the initiator of favorable local and systemic immune responses, and elective removal has been associated with poor survival in certain human cancers.10,16,17
Surgical Oncology
Surgery for Diagnosis
Surgery for Cure
Type
Plane of Dissection
Result
Intracapsular
Tumor removed in pieces or curetted, “debulking”
Macroscopic disease left behind
Marginal
Removal just outside or on pseudocapsule or reactive capsule, “shelled out”
Usually leaves microscopic disease
Wide
Tumor and capsule never entered, normal tissue surrounds specimen
Possible skip lesions
Radical
Entire compartment or structure removed (e.g., amputation)
No local residual cancer
Lymph Node Removal
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine