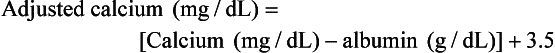5 The causes of PNSs are quite variable; they are usually caused by the production of small molecules (e.g., hormones, cytokines, or peptides) that are released into the circulation to cause effects at distant sites or by immune cross-reactivity between malignant and normal tissues. Some PNSs are due to functional mutations that result in overexpression of the small molecule in question, whereas many nonendocrine PNSs have no known etiology. PNSs are recognized commonly in both human and companion animal cancer patients.1 Box 5-1 summarizes the most common PNSs of dogs and cats and the tumors associated with them. An important systemic effect of cancer in animals is profound malnutrition and wasting. The weight loss and metabolic alterations observed in cancer patients despite adequate nutritional intake are termed cancer cachexia, whereas alterations observed as the result of poor nutritional intake are termed cancer anorexia. The clinical outcome of either cancer cachexia and/or anorexia is a progressive wasting (Figure 5-1). The weight loss endured by these patients is more than a simple cosmetic abnormality as human patients with cancer cachexia can have significantly reduced survival times, and many patients are unable to undergo appropriate therapy because of their poor clinical status. Cancer cachexia occurs frequently in human oncology, with estimated incidences from 40% to approximately 90% of hospitalized patients.2,3 Importantly, cancer cachexia accounts for approximately 20% of cancer deaths.4,5 The incidence of cancer cachexia in veterinary oncology patients is presently unknown, although this author believes that an estimated incidence of cancer cachexia in dogs is realistically 10% or less. For example, only 4% of dogs presenting to an academic oncology center were found to have cachexia, although referral bias likely results in some reporting artifact.6 The metabolic alterations associated with this PNS usually occur before weight loss is detected in both human and veterinary cancer patients.3,7–11 These metabolic alterations may last for some time after the patient is tumor free making it difficult for the clinician to reverse the weight loss.8,10 A plasmid-DNA–mediated approach utilizing growth hormone–releasing hormone (GHRH) in dogs appears to increase insulin-like growth factor-1 (IGF-1) levels (a measure of GHRH activity) and may represent a mechanism for attenuating cancer cachexia.12–15 In the clinical evaluation of a veterinary patient for the possibility of cancer cachexia/anorexia, a detailed history and physical examination are crucial. The prognostic importance of the presence of cancer cachexia in human cancer patients cannot be overstated because many studies show that this PNS is the only or one of very few independent multivariate negative prognostic factors for a variety of malignancies.2,3 A more detailed discussion of this syndrome is found in Chapter 15, Section B. Protein-losing enteropathy (PLE) is a syndrome whereby excessive serum proteins are lost into the gastrointestinal (GI) tract, leading to hypoproteinemia.16 The hypoproteinemia seen in cancer patients can be due to impaired synthesis and/or increased loss into the GI tract or urine (see Renal Manifestations of Cancer later in this chapter). Once the loss of proteins becomes greater than the body’s ability to synthesize them, serum protein levels begin to decrease. The half-life of many serum proteins is long, and patients with hypoproteinemia caused by PLE or some other cancer-related protein loss may represent long-standing protein loss.2,16 PLE is thought to result from an increase in mucosal serum protein permeability because of mucosal erosion, ulceration, or lymphatic obstruction. The diagnosis of PLE is made by noting hypoproteinemia on serum chemistry evaluation with subsequent exclusion of severe malnutrition and liver disease. Confirmation of the diagnosis is made in humans with PLE by alpha-1-antitrypsin detection,17 but this methodology has not been validated in veterinary medicine.18 In addition, nuclear scintigraphy appears to be a reliable methodology for the diagnosis of PLE.19 The incidence of PLE as a PNS is unknown in veterinary medicine but is likely to be rare. The treatment for PLE consists of treating the primary malignancy; however, those patients with a lymphangiectasia-related PLE may also be treated with medium-chain triglycerides that do not undergo transport by intestinal lymphatics. The most common cause of PNS-associated gastroduodenal ulceration is mast cell tumor (MCT). The excess histamine seen in MCTs stimulates gastric H2 receptors, leading to increased gastric acid secretion. Clinical manifestations of mucosal damage and/or ulceration with gastric vessel thrombosis occur in association with gastric hyperacidity. Plasma histamine concentrations are elevated in approximately 75% of dogs with macroscopic MCT, although only 30% have GI signs.20,21 Abnormally elevated plasma histamine concentrations have also been found to be a negative prognostic factor in dogs with MCT.21 Symptomatic therapies such as proton-pump inhibitors, H2 blockers, misoprostol, sucralfate, and rehydration may be helpful in combating PNS-associated gastroduodenal ulceration. MCTs are covered in greater detail in Chapter 20. An additional cause of PNS-associated gastroduodenal ulceration is gastrinoma (gastrin-secreting non–islet cell pancreatic tumor). Although these tumors are relatively rare, they have been reported in both dogs and cats.22–26 Gastrinomas can be associated with vomiting, lethargy, anorexia, blood loss, and abdominal pain. Many of these features are also seen in humans with gastrinoma-related Zollinger-Ellison syndrome. Gastrinomas are covered in greater detail in Chapter 25. The most common cause of hypercalcemia in the dog is cancer. A variety of tumors have been associated with hypercalcemia of malignancy (HM), and neoplasia is diagnosed in approximately two-thirds of dogs27,28 and one-third of cats with hypercalcemia.29 Lymphoma is the most common cause of HM (10% to 35% occurrence). Other tumor types associated with HM in dogs and cats include anal sac apocrine gland adenocarcinoma (≥25%), thyroid carcinoma, multiple myeloma (20%), bone tumors, thymoma, squamous cell carcinoma, mammary gland carcinoma/adenocarcinoma, melanoma, primary lung tumors, chronic lymphocytic leukemia, renal angiomyxoma, and parathyroid gland tumors.30–40 The causes of HM are varied and include ectopic production of parathormone (PTH) or PTH-related peptide (PTH-rp) by the tumor, extensive and usually multifocal lytic bone metastases, primary hyperparathyroidism, tumor-associated prostaglandins (PGE1/2), interleukin-1-β (IL-1β, previously known as osteoclast-activating factor [OAF]), transforming growth factor-β (TGF-β), and receptor activator of nuclear factor kappa-B ligand (RANKL).2,34,41–46 Interestingly, TGF-β1 regulates the mRNA stability of PTH-rp.47 The HM seen in lymphoma and anal sac apocrine gland adenocarcinoma is commonly caused by tumor-associated PTH-rp.48,49 PTH-rp is a 16-kDa protein with significant sequence identity to PTH, suggesting it may act and function like PTH. In addition to HM, other hypercalcemia differential diagnoses include “lab error” (lipemia and hemolysis), acute renal failure, hypervitaminosis D, hypoadrenocorticism, and granulomatous disease. When HM is diagnosed, appropriate steps for identification of the underlying neoplasm cause are necessary, and if azotemia accompanies HM, appropriate therapy to support renal function should be instituted quickly. The diagnostic evaluation of HM should begin with procedures used in the staging of lymphoma (as outlined in Chapter 32, Sections A and B) in addition to a rectal palpation and examination for anal sac apocrine gland adenocarcinoma. If these diagnostics do not confirm the specific cause of the HM, then the aforementioned hypercalcemia differential diagnoses should be considered and appropriately pursued. Dogs and cats with HM will typically have low PTH and high PTH-rp concentrations; however, the cause of the HM can usually be delineated with appropriate diagnostics before the return of PTH/PTH-rp assay results and such tests may not be routinely needed. Since HM is a potential medical emergency, the primary goal is elucidation of the underlying cause in order to institute the appropriate therapy for the specific tumor. Symptomatic therapy must be judiciously utilized while searching for the underlying cause of the HM. The premature administration of symptomatic therapy that includes the use of corticosteroids prior to the confirmation of the cause of the HM can have serious consequences. If lymphoma is the underlying cause of the HM, the use of corticosteroids may interfere with the ability to confirm a diagnosis, necessitating either additional diagnostics and/or waiting to determine if the lymphoma reappears after glucocorticoid withdrawal. In addition, glucocorticoids may induce resistance to other chemotherapy agents with a decrease in the ability to induce a complete remission, as well as a decrease in the length of survival.50 Therefore the use of corticosteroids in cases of undiagnosed hypercalcemia is strongly discouraged. Symptomatic therapies that promote external loss of calcium, increase renal excretion of calcium, and inhibit bone reabsorption may be utilized in HM patients. The severity of clinical signs and associated hypercalcemia determine the preferred therapy (Box 5-2). The use of 0.9% NaCl intravenously (IV) is recommended for management of existing dehydration and to expand the extracellular fluid volume, increase GFR, increase calciuresis and natriuresis, and decrease calcium reabsorption by the kidneys. Once rehydrated, the loop diuretic furosemide with continued normosaline diuresis can be utilized to potently inhibit calcium reabsorption in the ascending loop of Henle. If the cause of the HM is determined, corticosteroids can be extremely effective as adjunct therapy for HM by their inhibition of PGE, OAF (IL-1β), vitamin D, and intestinal calcium absorption. Corticosteroids can also be cytotoxic to lymphoma cells, the most common cause of HM. The most common therapies utilized in the treatment of HM are outlined in Box 5-2. In rare cases that are unresponsive to these symptomatic therapies and treatment of the underlying cause, other treatments such as calcitonin, bisphosphonates, or gallium nitrate may be utilized.51 Bisphosphonates have become the standard of therapy for human nonhumoral HM because of their potent inhibition of bone resorption without affecting tubular calcium reabsorption.52 The use of bisphosphonates in dogs and cats appears to be a promising treatment for hypercalcemia but requires additional study.53,54 In the future, treatment options for refractory HM may include osteoprotegerins, more potent bisphosphonates, anti-PTH-rp antibodies, noncalcemic calcitriol analogs, distal tubule calcium reabsorption inhibitors, RANKL antagonists, and new bone resorption inhibitors.52,55 The most common cause of cancer-induced hypoglycemia (<65 to 70 mg/dL serum glucose) in the dog is insulinoma (beta-islet cell tumor), and the reader is directed to Chapter 25 for a discussion of insulinomas.56 Nonislet cell tumors can also serve as sources of ectopic hormone production with resultant hypoglycemia in dogs and humans. Nonislet cell tumors with PNS hypoglycemia have been most commonly associated with hepatocellular carcinomas; however, lymphoma, hemangiosarcoma, oral melanoma, hepatoma, plasma cell tumor, multiple myeloma, smooth muscle tumors (leiomyoma and leiomyosarcoma), mammary tumors, renal tumors, and salivary gland tumors have also been reported.57–62 The hypoglycemia of extrapancreatic tumors has interestingly been associated with low insulin levels, whereas pancreatic beta-islet cell tumors (insulinomas) induce hypoglycemia by excessive circulating insulin levels. Nonislet cell tumors may induce hypoglycemia by increased tumor utilization of glucose, decreased hepatic glycogenolysis or gluconeogenesis, or the secretion of insulin or IGF-1 and IGF-2. Additional mechanisms include upregulation of insulin receptors, increased insulin binding by M proteins in myeloma, and increased production of somatomedins.2,63 The differential diagnoses of hypoglycemia include insulinoma, nonislet cell tumor hyperinsulinism, nonislet cell tumor, hypoadrenocorticism, starvation, sepsis, liver dysfunction, and laboratory error (lack of timely serum separation). Tumors associated with extrapancreatic hypoglycemia are often extremely large and therefore radiographs/ultrasound of the abdomen and/or thorax may be helpful. Exploratory laparotomy may be necessary if a space-occupying mass is not identified. Provocative testing via glucagon or glucose tolerance testing may be useful in cases when the diagnosis is uncertain. The reader is directed to Chapter 25 for an extensive discussion of the clinical consequences, diagnosis, and therapy of tumor-induced hypoglycemia. Syndrome of inappropriate secretion of ADH (SIADH) is a PNS widely recognized in lung, head, neck, and many other tumors in humans1,2; however, it continues to be essentially unrecognized in veterinary oncology. SIADH has been reported in dogs associated with heartworm disease, congenital hydrocephalus, and granulomatous amebic meningoencephalitis.64–66 In addition to PNS-associated SIADH, chemotherapy agents and other drugs (vincristine, cyclophosphamide, cisplatin, thiazides, morphine, and chlorpropamide), pulmonary and/or central nervous system (CNS) infections, and a variety of other conditions can cause SIADH.67,68 The initial finding in SIADH patients is hyponatremia. In addition to hyponatremia, serum hypo-osmolarity, hypernatriuresis, urine hyperosmolarity, and euvolemia with normal renal, thyroid, and adrenal function is noted.2,67 Although most human SIADH patients are asymptomatic, clinical signs can develop due to hyponatremia that result in CNS signs such as fatigue, anorexia, confusion and potentially seizures. The treatment of choice for PNS-associated SIADH is removal of the underlying cause. In addition, water restriction, demeclocycline (ADH antagonist), and hypertonic sodium chloride may be useful in SIADH cases.69 The ectopic production of adrenocorticotropic hormone (ACTH) or ACTH-like substances is the second most common PNS reported in humans.2 This PNS is associated with small cell lung tumors, pancreatic tumors, and a wide variety of other human tumors.2,67 In the dog, this PNS is reported to occur in primary lung tumors and a single case of an abdominal neuroendocrine tumor.70,71 The predominant active molecules in this PNS are ACTH, ACTH precursors, endorphins, enkephalins, and melanocyte-stimulating hormone (MSH).2,67 All result in excessive production of steroids from the adrenal glands resulting in clinical signs similar to those seen in hyperadrenocorticism (Cushing’s disease). Invariably, tumors that cause this PNS are dexamethasone insuppressible. The diagnosis of this PNS is made by the concomitant presentation of Cushing’s-like signs with an abnormal dexamethasone suppression test and a localizable tumor. The treatment of choice is removal of the underlying cause by surgical extirpation of the tumor. When necessary, the medical therapy for this PNS centers on inhibiting cortisol production by mitotane or ketoconazole. The use of selegiline (Anipryl) in the management of this PNS in dogs has not been evaluated to date in veterinary medicine. Paraneoplastic hypocalcemia and hyperglycemia are extremely rare. Tumors associated with lytic bone metastases and tumors that secrete calcitonin (medullary carcinoma of the thyroid) are the most common causes of human PNS hypocalcemia.2 A variety of nonthyroid cancers such as breast cancer, GI cancer, carcinoids, and lung cancer in humans have been reported to secrete calcitonin.72 Most cases of PNS hypocalcemia are asymptomatic, although human patients may have neuromuscular irritability and/or tetany. A gingival vascular hamartoma in a 4-month-old kitten has been reported to be associated with PNS hyperglycemia, and the hyperglycemia resolved within 24 hours on removal of the tumor.73 Treatment of choice for either PNS is eradication of the primary tumor whenever possible, calcium infusion in severe hypocalcemia cases, and diabetes-like support for severe hyperglycemia cases. Monoclonal gammopathies can be common in animals and people with cancer and they are termed M-component disorders.1,2,74–76 The hypergammaglobulinemia seen as a PNS is due to the excessive production of proteins from a monoclonal line of immunoglobulin (Ig)-producing plasma cells or lymphocytes. When production of these Igs, partial Igs, heavy chains, and/or light chains becomes extreme, clinical signs of hyperviscosity (ataxia, depression, dementia, cardiac disease and/or failure, seizures, and coma), tissue hypoxia, bleeding (poor platelet aggregation, platelet coating with Igs, and release of platelet factor III), and/or ocular disorders (e.g., papilledema, retinal hemorrhage, detachment) may occur. These proteins may be identified by performing a protein electrophoresis on the serum and/or urine.76 Similarly, light chain production may be detected in the urine as Bence-Jones proteins. In addition to the hypergammaglobulinemia PNS seen in plasma cell tumors (multiple myeloma and extramedullary plasmacytoma), lymphomas, lymphocytic leukemias, and primary macroglobulinemia can also cause this PNS.75,76 Further discussion of plasma cell tumors, myeloma and lymphoma/leukemia can be found in Chapter 32. Anemia is one of the most common PNSs seen in veterinary and human oncology. Approximately 20% to 25% of human cancer patients have PNS anemia, and although the exact incidence of PNS anemia in veterinary oncology is unknown, it is thought to be a significant problem.77 There are numerous possible causes for PNS anemia in veterinary oncology patients, and the vast majority are due to either anemia of chronic disease (ACD), immune-mediated hemolytic anemia (IMHA), blood loss anemia, or microangiopathic hemolytic anemia (MAHA). ACD is extremely common in veterinary and human oncology patients with disseminated and/or metastatic tumors. This anemia is due to disordered iron storage and metabolism, shortened red blood cell (RBC) lifespan, and occasionally decreased bone marrow response.77 The anemia seen in ACD is normocytic/normochromic, and evaluation of the bone marrow does not suggest significant problems with cellularity. Treatment of choice is removal of the tumor. IMHA can be triggered by tumors in animals and humans. Immune mechanisms then result in the premature destruction of RBCs.78 The diagnosis of PNS IMHA is typically established by a Coombs’ slide agglutination test, and many patients will have concurrent spherocytosis and a regenerative anemia. The treatment of choice is removal of the tumor; however, if this is not immediately possible, the use of immunosuppressive dosages of prednisone (1 to 2 mg/kg daily to two times a day by mouth [BID PO]) may be indicated if a diagnosis has been established. Similar to non-PNS IMHA, the use of additional agents such as azathioprine (1 to 2 mg/kg daily for 4 to 7 days, then 0.5 to 1 mg/kg every 48 hrs PO), cyclosporine, cyclophosphamide, and others may be necessary for complicated IMHA cases.78–81 Blood loss anemia can be a sequela to many types of cancer. Due to decreased hemoglobin content, the RBCs in blood loss anemia are microcytic/hypochromic. In addition, decreased serum iron, increased total iron-binding capacity, and poikilocytosis may also be noted.82,83 The blood loss may be readily apparent in some patients (e.g., bleeding splenic tumor or bleeding superficial tumor), whereas others may not have a readily identifiable source for the loss (e.g., GI tumors). The treatment of choice is removal of the tumor, although severe anemia may necessitate blood transfusions. The use of oral and/or injectable iron may be a useful adjunct therapy. MAHA is a secondary phenomenon to hemolysis and is typically due to fibrin deposition and/or endothelial damage.77 The most common causes of MAHA are PNS disseminated intravascular coagulation (DIC) and RBC shearing as the result of hemangiosarcoma.77,80 Schistocytosis and hemolysis are common indicators of ongoing MAHA. While any tumor can cause DIC and subsequent MAHA, hemangiosarcoma is most common.84 The treatment of choice is removal of the tumor; however, additional ancillary treatments such as aggressive supportive therapy and transfusions may be useful. Chemotherapy-induced anemia can be quite common in people because of more aggressive chemotherapy protocols2; however, this is rarely seen in veterinary patients.77,82,83 The degree of anemia seen in veterinary cancer patients undergoing chemotherapy is generally mild, with typical packed cell volumes hovering in the 28% to 32% range for dogs and 24% to 28% range for cats. This anemia rarely necessitates therapy and resolves on discontinuation of the chemotherapy protocol. A relatively uncommon cause of PNS-associated anemia is myelophthisis (bone marrow invasion/crowding out), and it is most commonly caused by leukemias.2 Another uncommon cause of PNS-associated anemia is bone marrow hypoplasia resulting from hyperestrogenism. Sertoli cell tumors in the male dog and granulosa cell tumors of the female dog are commonly associated with hyperestrogenism, and anemia is a common presenting feature.85–87 Erythrocytosis is a relatively uncommon PNS. Tumors that have been associated with PNS erythrocytosis include renal tumors (primary and secondary), lymphoma (including renal origin), lung or liver tumors, cecal leiomyosarcoma, nasal fibrosarcoma, and transmissible venereal tumor (TVT).62,88–93 The erythrocytosis seen in cancer patients can be due directly to overproduction of erythropoietin, indirect excess erythropoietin from renal hypoxia, or increased production of hypoxia-inducible factors such as HIF-1.94 Interestingly, some tumor suppressor genes (TSG) are important proteosomal regulators of HIF-1, which may explain some of the vascular diseases seen in human patients with certain TSG mutations.95 Other differential diagnoses for polycythemia include arteriovenous shunts, severe dehydration, hyperadrenocorticism, polycythemia vera (primary polycythemia), and a variety of cardiac and/or pulmonary diseases. It is important to differentiate primary and secondary causes of erythrocytosis. Polycythemia vera is a myeloproliferative disorder resulting in clonal proliferation of RBCs with splenomegaly and possibly pancytosis most commonly associated with acquired recurrent mutation in JAK2.1,2,96 Secondary polycythemia results from decreased arterial oxygen saturation. PNS erythrocytosis is best treated by removal of the erythropoietin-producing tumor whenever possible; phlebotomy can be a useful temporary adjunct therapy. Unfortunately, the volumes typically needed for a therapeutic phlebotomy in PNS erythrocytosis cases necessitates administration of fluids and potentially readministration of plasma. The use of hydroxyurea (40 to 50 mg/kg divided BID PO)91 as a chemotherapeutic agent for polycythemia vera has been previously recommended; however, this author has noted limited benefit with use of this agent.
Paraneoplastic Syndromes
Gastrointestinal Manifestations of Cancer
Protein-Losing Enteropathy
Gastroduodenal Ulceration
Endocrinologic Manifestations of Cancer
Hypoglycemia
Syndrome of Inappropriate Secretion of Antidiuretic Hormone
Ectopic Adrenocorticotropic Hormone Syndrome
Hypocalcemia/Hyperglycemia
Hematologic Manifestations of Cancer
Anemia
Erythrocytosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine