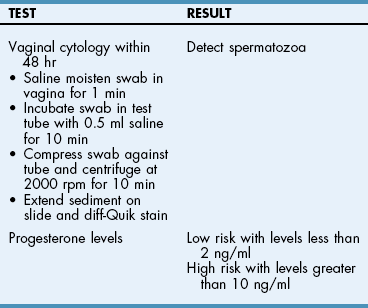
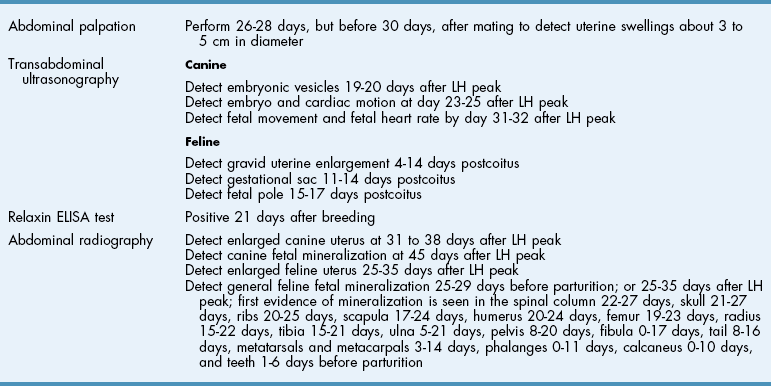
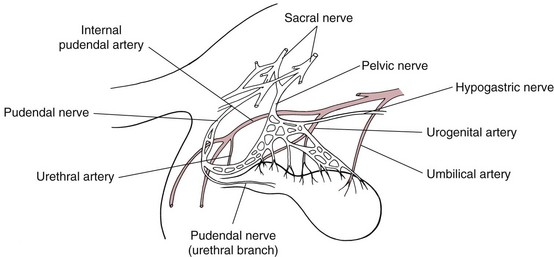
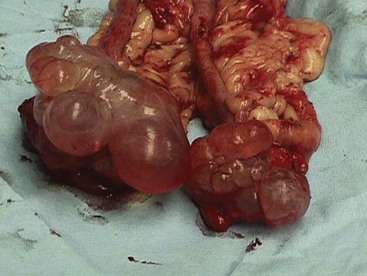
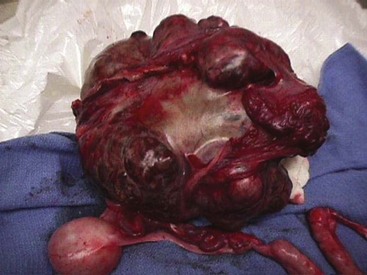
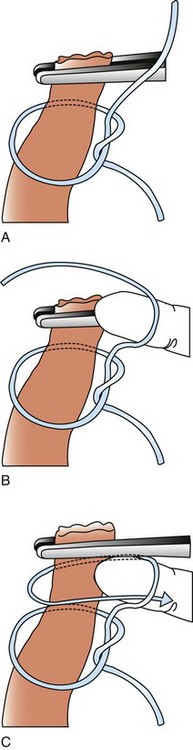
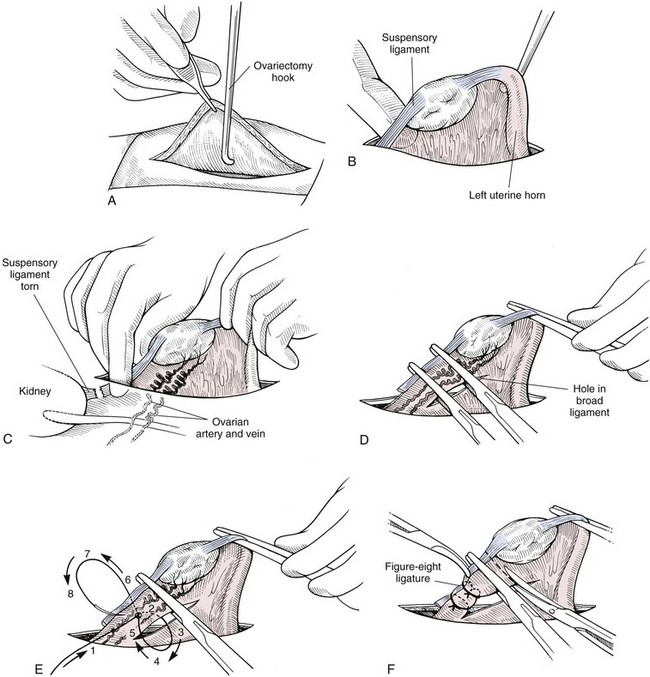
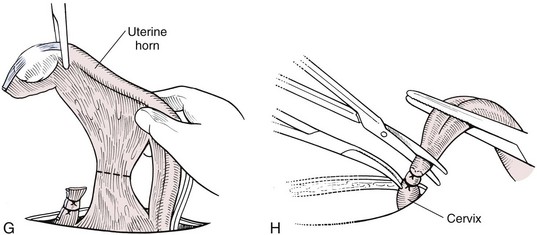
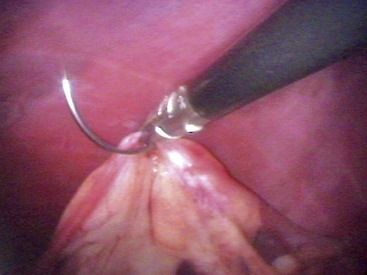
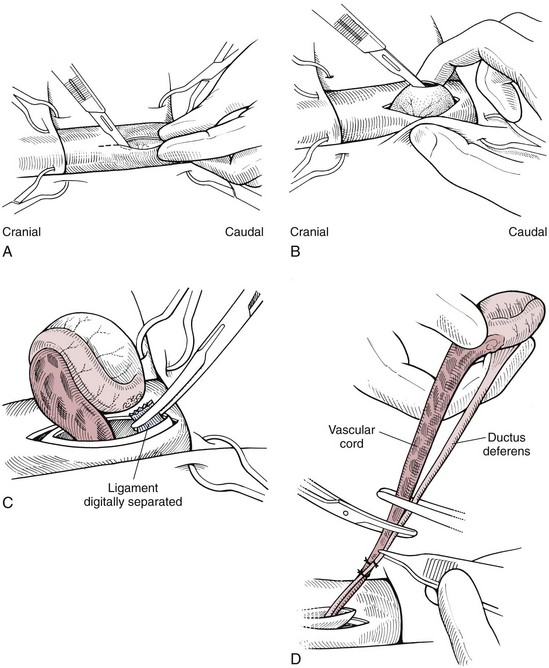
Chapter 27 General Principles and Techniques Reproductive surgery encompasses a variety of techniques designed to alter the animal’s ability to reproduce, aid parturition, or treat or prevent disease of the reproductive organs (Box 27-1). The primary indication for reproductive tract surgery is to limit reproduction, but it may also be done to relieve dystocia, prevent or treat tumors influenced by reproductive hormones (e.g., mammary tumors, testicular tumors, and perianal adenomas), control certain diseases of the reproductive tract (e.g., pyometra, metritis, prostatitis, tumor, and prostatic abscessation), and help stabilize systemic disease (e.g., diabetes and epilepsy). Neutering is performed in some animals to prevent or alter behavioral abnormalities and to reconstruct traumatized, diseased, or malformed tissue. Diagnosis of reproductive tract disease is based on history, clinical signs, physical examination, diagnostic imaging (e.g., radiographs, ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and bone scan), endoscopy, cytology, microbiology, hormonal assay, hematology, serum biochemistry profile, urinalysis, and/or other laboratory results. History and clinical signs of animals needing reproductive surgery depend on the gender and disease. Most animals brought in for elective reproductive surgery (i.e., castration, OVE, or OHE) are healthy. Asymptomatic animals with neoplasia may have a mass found incidentally by the owner. Those with genital tract infections may be severely ill and have fever, toxemia, incontinence, and/or obstruction. Prostatic disease is common in male dogs; clinical signs may be nonspecific (i.e., fever, malaise, vomiting, dehydration, caudal abdominal pain, and/or gait abnormalities). Hematuria caused by reflux of blood from the prostatic urethra into the bladder and/or concurrent urinary tract infection may occur. Urethral discharge (preputial/urethral drip) is caused by passive release of blood, pus, or prostatic fluid into the prostatic urethra. Stranguria occurs when the prostate compresses or obstructs the urethra, or there is inflammation caused by urinary tract infection. Urinary incontinence (see p. 771) may be caused by prostatic impingement on the pelvic nerves. Prostatic enlargement compressing the rectum can cause tenesmus or smaller stool diameter. Constipation may occur secondary to obstruction or pain during defecation. If masses are identified, cytology of aspirates or discharges may help differentiate inflammation from neoplasia. Vaginal, uterine, or mammary gland cultures are recommended if infection is suspected. Vaginal cytology should be consistent with the bitch’s estrus cycle. The normal vaginal flora includes numerous aerobic and anaerobic bacteria (Box 27-2). Pure growth of bacteria from vaginal specimens can be normal unless accompanied by signs of reproductive tract disease. Persistent vaginal discharge is an indication for brucellosis testing. Assessment of hormone levels is occasionally helpful in diagnosing female reproductive problems. In female dogs, estradiol levels are ≤10 pg/ml during late anestrus, ≥10 pg/ml at onset of proestrus, and 50 to 100 pg/ml at late proestrus. Progesterone levels are 0.5 to 1 ng/ml during anestrus and proestrus, 2 to 5 ng/ml at luteinizing hormone (LH) surge; levels peak at 15 to 90 ng/ml 15 to 30 days later, and slowly decline thereafter to ≤2 ng/ml in pregnant (mean 63 days) or nonpregnant diestrus (50 to 100 days) bitches (Table 27-1). Progesterone greater than 2 ng/ml is necessary to maintain pregnancy. Progesterone must fall below 2 ng/ml to initiate parturition; a drop in progesterone levels to less than 1 to 2 ng/ml for 2 consecutive days will typically terminate pregnancy. Measuring LH helps distinguish between neutered and intact bitches, or queens, ovarian remnant syndrome, or administration of exogenous sex steroids. Knowing the time of the LH surge (greater than 1 ng/ml) is helpful in determining ideal breeding times and predicting parturition. Thoracic and abdominal radiographs and ultrasound, CT, or MRI images may be needed if reproductive tract tumors are suspected. Survey radiographs rarely identify the normal nongravid uterus. Radiographically, an enlarged gravid uterus can be detected within 31 to 38 days and fetal skeletal mineralization by 45 days after the LH peak (within a mean of 0.5 day of onset of estrus) (Table 27-2). In cats it is difficult to know when the LH peak has occurred because behavioral estrus is variable (1 to 21 days); therefore, fetal skeletal mineralization, which is first detected at 25 to 29 days before parturition, is used to predict when parturition will occur. Ovarian masses and follicular changes can be detected in the periovulatory period using ultrasonography. The normal, nongravid uterus can be identified using a high-frequency (>10 MHz) transducer. Using a fluid-distended urinary bladder as an acoustic window may reveal fluid-distended horns and a thickened uterine wall. Pregnancy and fetal viability can be detected ultrasonographically as early as 19 to 20 days after the LH surge in dogs or 15 to 17 days postcoitus in cats, but pregnancy diagnosis by ultrasound is uncomplicated at 30 days of gestation (see Table 27-2) (Davidson and Baker, 2009). Ultrasonographic signs of abortion or lack of fetal viability include changes in fetal anatomy, placental detachment, or fetal resorption; however, serial evaluations may be necessary. Ultrasonography can also detect uterine cysts, masses, or fluid; premature placental separation; and a thickened uterine wall. Cystometrograms and urethral pressure profiles may occasionally help assess dogs with urinary incontinence (see Chapter 26) but are rarely performed. Histologic evaluation is often necessary to confirm the diagnosis of female reproductive disorders and allow a more accurate prognosis with most conditions. Numerous anesthetic protocols may be used for elective surgery in healthy animals. As with any other surgery, a balanced technique is recommended in order to provide adequate anesthesia and analgesia. Suggested anesthetic protocols for healthy animals undergoing elective surgery are provided in Table 27-3. Also, refer to Chapter 12 for a discussion of the variety of medications that may be used on healthy animals (see Boxes 12-1 and 12-2 on pp. 136 and 138, respectively, and see Tables 12-2 to 12-4 and 12-6 on pp. 138, 141, 142, and 152, respectively). Anesthetic Considerations in Healthy Patient for Routine Surgery *Monitor for hyperthermia in cats. †Buprenorphine is a better analgesic than morphine in cats. §Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the U.S. Patients requiring cesarean section are often greater anesthetic risks because of hypovolemia, hypoglycemia, and hypocalcemia. The pregnant dog or cat has numerous metabolic changes including increased circulating blood volume, cardiac output, heart rate, and oxygen consumption. Additionally, there are decreases in packed cell volume, lung volumes, and gastric emptying. The primary anesthetic goal is to adequately and safely anesthetize the mother without endangering the fetuses. Drugs that depress the mother also depress the fetus. Therefore, minimal preoperative medications are used. The patient should be rehydrated if necessary. Preoxygenating is recommended in most pregnant patients for approximately 3 minutes. The patient is then rapidly induced and intubated. Propofol is an excellent induction agent because it has a rapid onset with no residual neonatal depression. Etomidate is also effective. Fentanyl may be given with induction and titrated to effect prior to the removal of puppies or kittens. Maintenance should be done with isoflurane or sevoflurane with oxygen. Nitrous oxide crosses the placental barrier but lower levels are found in the fetus than in the mother. Nitrous oxide at 50% is less likely to cause neonatal hypoxemia. If time allows, a fentanyl epidural will assist in decreasing the mother’s anesthetic requirement. This can be accomplished immediately after induction and before prepping of the abdomen. Before making the skin incision, a lidocaine line block may be used in cats (at reduced dosages) and dogs. It is recommended to minimize the medications given prior to the removal of the fetuses. Once this is completed, other opioids (i.e., hydromorphone, morphine, buprenorphine) and ketamine may be given. Have glycopyrrolate and naloxone available in the operating room. Give glycopyrrolate if the mother becomes bradycardic because of uterine manipulation. Naloxone may be given at doses of one to two drops sublingually to the neonates if respiratory depression secondary to the fentanyl is suspected. Suggested anesthetic protocols for cesarean section are provided in Table 27-4. See pp. 798-800 for techniques for cesarean section and neonatal care. Anesthetic Considerations in the Patient Undergoing a Cesarean Section *Monitor for hyperthermia in cats. †Buprenorphine is a better analgesic than morphine in cats. ‡Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the United States. Perioperative antibiotics are not necessary for elective OHE or castration. Antibiotic choice should be based on culture and susceptibility or on expected pathogens in patients with pyometra, metritis, or bacterial prostatitis. Until culture results are available, antibiotics used to treat pyometra should be efficacious against Escherichia coli because this is the most common pathogen. Aminoglycosides are nephrotoxic and should be avoided when possible because of the renal dysfunction seen in pyometra. Choice of prophylactic antibiotics for surgery involving tumors or trauma depends on the patient’s condition and surgeon’s preference (see Chapter 9). Antibiotic selection for prostatic diseases should be based on culture results and expected blood-prostate barrier penetration. Antibiotics should be lipid-soluble (usually nonionized), nonprotein bound, and have a high pKa (degree of drug ionization; high pKa = more basic). Those with a high degree of lipid solubility are best at crossing the blood-prostate barrier. Antibiotics with a high pKa are less ionized at physiologic conditions and concentrate in the prostate. Prostatic infections produce acidic prostatic fluid that helps trap antibiotics in the fluid. Basic antibiotics that concentrate in the prostate include erythromycin, clindamycin, and trimethoprim (Box 27-3). Enrofloxacin and doxycycline achieve high prostatic fluid concentrations and are effective against some resistant Gram-negative urogenital pathogens. The female reproductive tract includes the ovaries, oviduct, uterus, vagina, vulva, and mammary glands. The ovaries are located within a thin-walled peritoneal sac; the ovarian bursa is located just caudal to the pole of each kidney. The uterine tube or oviduct courses through the wall of the ovarian bursa. The right ovary lies further cranially than the left. The right ovary lies dorsal to the descending duodenum, and the left ovary lies dorsal to the descending colon and lateral to the spleen. Medial retraction of the mesoduodenum or mesocolon exposes the ovary on each side. Each ovary is attached by the proper ligament to the uterine horn and via the suspensory ligament to the transversalis fascia medial to the last one or two ribs. The ovarian pedicle (mesovarium) includes the suspensory ligament with its artery and vein, ovarian artery and vein, and variable amounts of fat and connective tissue. Canine ovarian pedicles contain more fat than feline ovarian pedicles, making it more difficult to visualize the vasculature. The ovarian vessels take a tortuous path within the pedicle. Ovarian arteries originate from the aorta. The left ovarian vein drains into the left renal vein; the right vein drains into the caudal vena cava. The suspensory ligament is a tough, whitish band of tissue that diverges as it travels from the ovary to attach to the last two ribs. The broad ligament (mesometrium) is the peritoneal fold that suspends the uterus. The round ligament travels in the free edge of the broad ligament from the ovary through the inguinal canal with the vaginal process. The uterus has a short body and long narrow horns. The uterine arteries and veins supply blood to the uterus. The cervix is the constricted caudal part of the uterus and is thicker than the uterine body and vagina. It is oriented in a nearly vertical position with the uterine opening dorsally. The vagina is long and connects with the vaginal vestibule at the urethral entrance. The clitoris is broad, flat, vascular, infiltrated with fat, and lies on the floor of the vestibule near the vulva. The clitoral fossa is a depression on the floor of the vestibule that is sometimes mistaken for the urethral orifice. The vulva is the external opening of the genital tract. The vulvar lips are thick and form a pointed commissure. The constrictor vulvae and constrictor vestibule muscles encircle the vulva and vestibule. See page 812 for a description of the anatomy of the mammary glands. The major components of the male genital tract are the testicles, penis, and prostate. The prostate gland completely surrounds the neck of the bladder and beginning of the urethra. In dogs less than 4 years of age, the prostate is usually located in the pelvic cavity at the brim of the pubis. The prostate begins to enlarge at puberty, becoming intra-abdominal in location. It varies greatly in size at maturity. The prostate is encapsulated by fibromuscular tissue and is bilobate with a prominent mid-dorsal sulcus. The dorsal sulcus continues into the prostatic parenchyma as the median septum. The ventrolateral surfaces of the prostate are covered by a fat pad. The parenchyma is lobulated with tubuloalveolar glands that empty through small ducts (12 to 20) into the prostatic urethra. The ductus deferens enters the craniodorsal surface of the prostate and courses caudoventrally to enter the urethra at the colliculus seminalis. The blood and nerve supply (pelvic and hypogastric nerves) are located in the lateral pedicles (peritoneal reflection), entering the prostate at the 10 o’clock and 2 o’clock positions when viewed in a transverse plane. The prostatic arteries originate from the urogenital artery (branch of internal iliac artery) and supply branches to the ductus deferens, urethra, urinary bladder, ureters, and rectum. The hypogastric (sympathetic) and pelvic (parasympathetic) nerves follow the vasculature and are essential for micturition and continence (Fig. 27-1). The pudendal nerve sends branches along the ventral surface of the urethra extending to the bladder neck. The pudendal nerve innervates the skeletal muscle of the external urethral sphincter. The iliac lymph nodes drain the prostate. In cats, bulbourethral glands are found caudal to the prostate at the ischial arch. Pediatric tissues are more fragile than adult tissues and must be handled gently. In young animals, 3-0 to 5-0 ligatures should be used. Early neutering delays growth plate closure by an average of 8 to 9 weeks, resulting in increased bone length in male and female dogs and cats. Infantile vulva and mammary glands or penis, prepuce, and os penis persist following early neutering. Bitches are at a greater risk to develop urinary incontinence if OHE is performed before 3 months of age (Box 27-4). Large-breed dogs neutered before 6 months of age are at increased risk for development of excessive tibial plateau angle (Duerr et al, 2007). However, early neutering affects weight gain, daily food consumption, and activity level to the same degree as neutering after puberty. The traditional method of surgical sterilization of healthy female dogs, particularly in the United States, has been OHE; whereas OVE is practiced commonly in European countries. Evaluation of the scientific literature found no significant difference in postoperative complications between OHE and OVE, including the development of urethral sphincter mechanism incompetence, endometritis, and pyometra (van Goethem et al, 2006). In addition, uterine tumors are uncommon, and 85% to 90% are benign in nature. A recent prospective study found no difference in total surgical time, pain scores, or wound scores between dogs undergoing OVH versus OVE (Peeters and Kirpensteijn, 2011). However, OHE is technically more complicated and time consuming, whereas OVE can be performed quicker and through a smaller abdominal incision (or by laparoscopy) with less traction on the genital tract. Therefore, both OHE and OVE can be considered appropriate procedures for neutering healthy female dogs. The most common reason to perform OHE or OVE is to prevent estrus and unwanted offspring. Alternative methods of inhibiting reproduction are listed in Box 27-5. Other reasons for OHE include prevention of mammary tumors or congenital anomalies; prevention and treatment of pyometra, metritis, neoplasia (i.e., ovarian, uterine, or vaginal), cysts (Figs. 27-2 and 27-3), trauma, uterine torsion, uterine prolapse, subinvolution of placental sites, vaginal prolapse, and vaginal hyperplasia; and control of some endocrine abnormalities (i.e., diabetes and epilepsy) and dermatoses (e.g., generalized Demodex). Many technical variations of OHE have been described, including flank and laparoscopic approaches and the use of stapling equipment, ultrasonic scalpel, vessel sealing devices, transfixation ligatures, or Miller’s knots (Fig. 27-4). Only one technique for OHE is described here. Clip and surgically prepare the ventral abdomen from the xiphoid to the pubis. Identify the umbilicus, and visually divide the caudal abdomen into thirds. In dogs, make the incision just caudal to the umbilicus in the cranial third of the caudal abdomen. More caudal incisions make it difficult to exteriorize canine ovaries. In deep-chested dogs or in those with an enlarged uterus, extend the incision cranially or caudally to allow exteriorization of the tract without excessive traction. In prepubertal puppies, making the incision in the middle third of the caudal abdomen facilitates uterine body ligation. In cats, the body of the uterus is more caudal and difficult to exteriorize; therefore, make the incision in the middle third of the caudal abdomen. Make a 4- to 8-cm incision through skin and subcutaneous tissue to expose the linea alba. Grasp the linea alba or ventral rectus sheath, tent it outward, and make a stab incision into the abdominal cavity. Extend the linea incision cranial and caudal to the stab incision with Mayo scissors. Elevate the left abdominal wall by grasping the linea or external rectus sheath with thumb forceps. Slide the ovariectomy hook (e.g., Covault or Snook) with the hook against the abdominal wall, 2 to 3 cm caudal to the kidney (Fig. 27-5, A). Turn the hook medially to ensnare the uterine horn, broad ligament, or round ligament, and gently elevate it from the abdomen. Anatomically confirm the identification of the uterine horn by following it to either the uterine bifurcation or ovary. If the uterine horn cannot be located with the hook, retroflex the bladder through the incision and locate the uterine body and horns between the colon and bladder. With caudal and medial traction on the uterine horn, identify the suspensory ligament by palpation at the taut fibrous band at the proximal edge of the ovarian pedicle (Fig. 27-5, B). Stretch or break the suspensory ligament near the kidney without tearing the ovarian vessels to allow exteriorization of the ovary. To achieve this, use the index finger to apply caudolateral traction on the suspensory ligament while maintaining caudomedial traction on the uterine horn (Fig. 27-5, C). Make a hole in the broad ligament caudal to the ovarian pedicle. Place two or three Crile or Rochester-Carmalt forceps across the ovarian pedicle proximal (deep) to the ovary and one across the proper ligament of the ovary (Fig. 27-5, D). The proximal (deep) clamp serves as a groove for the ligature, the middle clamp holds the pedicle for ligation, and the distal clamp prevents backflow of blood after transection. When using two clamps, the ovarian pedicle clamp serves both to hold the pedicle and to make a groove for the ligature. Place a figure-eight ligature proximal to (below) the ovarian pedicle clamps (Fig. 27-5, E). Choose an absorbable suture material for ligatures (i.e., 2-0 or 3-0 polydioxanone [PDS], polyglyconate [Maxon], poliglecaprone 25 [Monocryl], glycomer 631 [Biosyn], or polyglactin 910 [Vicryl]). Begin by directing the blunt end of the needle through the middle of the pedicle, loop the suture around one side of the pedicle, then redirect the needle through the original hole from the same direction and loop the ligature around the other half of the pedicle. Securely tie the ligature. Remove one clamp or “flash” a single clamp while tightening the ligature to allow pedicle compression. Place a second circumferential ligature proximal to (below) the first to control hemorrhage that may occur from puncturing a vessel as the needle is passed through the pedicle. Some surgeons prefer to place the circumferential ligature or Miller’s knot (see Fig. 27-4) before the transfixing ligature to eliminate hemorrhage if a vessel is punctured during transfixation. Place a mosquito hemostat on the suspensory ligament near the ovary (Fig. 27-5, F). Transect the ovarian pedicle between the Carmalt and ovary. Open the ovarian bursa and examine the ovary to be certain that it has been removed in its entirety. Remove the Carmalt from the ovarian pedicle and observe for hemorrhage. Replace the Carmalt and religate the pedicle if hemorrhage is noted. Perform the identical procedure on the other side. Trace the uterine horn to the uterine body. Grasp the other uterine horn, and follow it to the opposite ovary. Place clamps and ligatures as just described. Make a window in the broad ligament adjacent to the uterine body and uterine artery and vein. Place a Carmalt across the broad ligament on each side and transect (Fig. 27-5, G). Apply a ligature around the broad ligament if the patient is in estrus or pregnant, or if the broad ligament is heavily infiltrated with vessels or fat. Apply cranial traction on the uterus, and ligate the uterine body cranial to the cervix. Place a figure-eight suture through the body using the point of the needle and encircling the uterine vessels on each side. Place a circumferential ligature nearer the cervix (Fig. 27-5, H). Place a Carmalt across the uterine body cranial to the ligatures. Grasp the uterine wall with forceps or mosquito hemostats cranial to the ligatures. Transect the uterine body, and observe for hemorrhage. Religate if hemorrhage is observed. Some surgeons place one to three Carmalt across the uterine body before ligation. In cats, clamps may cut rather than crush a friable or engorged uterus and cause transection before ligature placement. An alternative to ligatures is to use an ultrasonic scalpel, vascular sealer, or staples. Replace the uterine stump into the abdomen before releasing the hemostats or forceps. Close the abdominal wall in three layers (fascia/linea alba, subcutaneous tissue, and skin). Multiple variations of minimally invasive techniques for surgical sterilization have been described including intracorporeal versus laparoscopic-assisted OHE, OHE versus OVE, and differences in the number of portals utilized (one, two, or three). A three-port technique for laparoscopic-assisted OHE and a two-port technique for laparoscopic-assisted OVE are described here (Gower and Mayhew, 2008). Alternatively, place the laparoscope port just caudal to the umbilicus and a single instrument port midway between the umbilicus and pubis. Tilt the animal to one side and use a blunt probe or Babcock or Kelly laparoscopic forceps in the instrument port to retract the intestines medially and locate the ovary. Grasp the proper ligament of the ovary with forceps, and elevate the ovary away from the abdominal viscera but against the body wall. Introduce a large curved needle through the body wall near where the ovary is being held (Fig. 27-6). Direct the needle through the proper ligament under direct laparoscopic visualization, and then back through the body wall. Anchor the needle with a hemostat to keep the ovary suspended away from the abdominal viscera. Remove the laparoscopic forceps from the proper ligament, and replace this instrument with a bipolar vessel sealing device through the instrument port. Transect the suspensory ligament, ovarian vascular pedicle, and proper ligament. Tilt the animal in the opposite direction, and repeat the procedure on the remaining ovary. Remove each ovary from the abdominal cavity through the instrument port immediately following transection, or retain the first ovary against the body wall using the transabdominal needle until the entire procedure is complete to facilitate easier removal of both ovaries. Remove remaining instrumentation, complete evacuation of pneumoperitoneum, and close the incisions routinely. Position the patient in dorsal recumbency. Verify the presence of both testicles in the scrotum. Clip and aseptically prepare the caudal abdomen and medial thighs. Avoid irritating the scrotum with clippers or antiseptics. Drape the surgical area to exclude the scrotum from the field. Apply pressure on the scrotum to advance one testicle as far as possible into the prescrotal area. Incise skin and subcutaneous tissue along the median raphe over the displaced testicle (Fig. 27-7, A). Continue the incision through spermatic fascia to exteriorize the testicle. Incise the parietal vaginal tunic over the testicle (Fig. 27-7, B). Do not incise the tunica albuginea because this would expose the testicular parenchyma. Place a hemostat across the vaginal tunic where it attaches to the epididymis. Digitally separate the ligament of the tail of the epididymis from the tunic while applying traction with the hemostat on the tunic (Fig. 27-7, C). Further exteriorize the testicle by applying caudal and outward traction. Identify the structures of the spermatic cord. Individually ligate the vascular cord and ductus deferens, then place an encircling ligature around both. Many surgeons ligate the ductus deferens and pampiniform plexus together. Use 2-0 or 3-0 absorbable suture (e.g., polyglactin 910 [Vicryl], polydioxanone [PDS], poliglecaprone 25 [Monocryl], polyglyconate [Maxon], or glycomer 631 [Biosyn]) for ligatures. Alternatively, use hemostatic staples. Place a hemostat across the cord near the testicle. Grasp the ductus deferens with thumb forceps above the ligature, and transect both the ductus deferens and vascular cord between the hemostat and ligatures (Fig. 27-7, D). Inspect the cord for hemorrhage, and replace the cord within the tunic. Encircle the cremaster muscle and tunic with a ligature. Advance the second testicle into the incision, incise the fascial covering, and remove the testicle as described. Appose the incised dense fascia on either side of the penis with interrupted or continuous sutures. Close subcutaneous tissue with a continuous pattern. Appose skin with an intradermal, subcuticular, or simple interrupted suture pattern.
Surgery of the Reproductive and Genital Systems
Preoperative Management
History
Females
Clinical pathology
Hormone concentrations
Diagnostic imaging
Other tests
ANESTHEsia
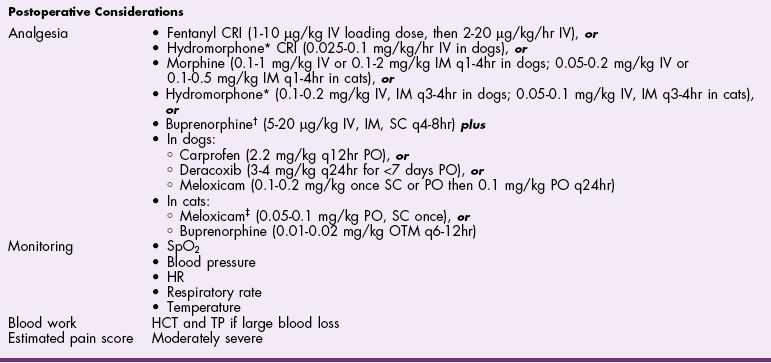
![]() TABLE 27-3
TABLE 27-3


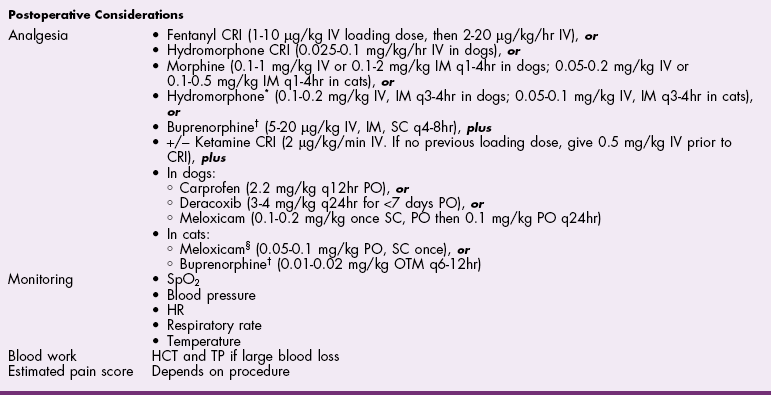
![]() TABLE 27-4
TABLE 27-4


Antibiotics
Surgical Anatomy
Male Reproductive Tract
Surgical Technique
Ovariohysterectomy/Ovariectomy
Laparoscopic-Assisted Ovariohysterectomy or Ovariectomy
Orchiectomy
Canine castration
Open Prescrotal Castration
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree