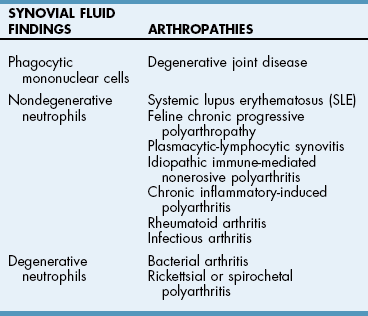
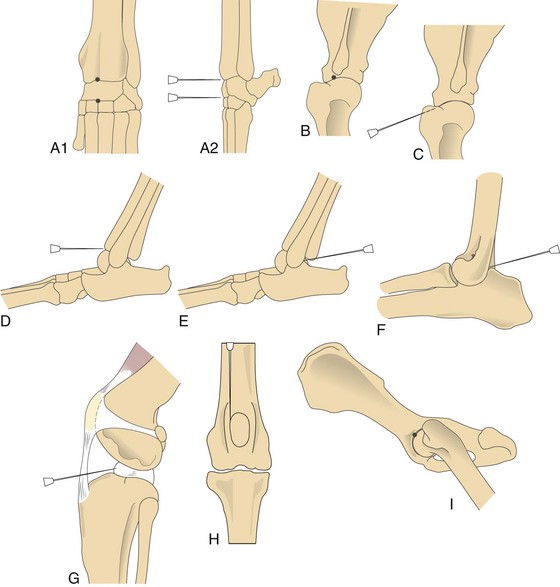
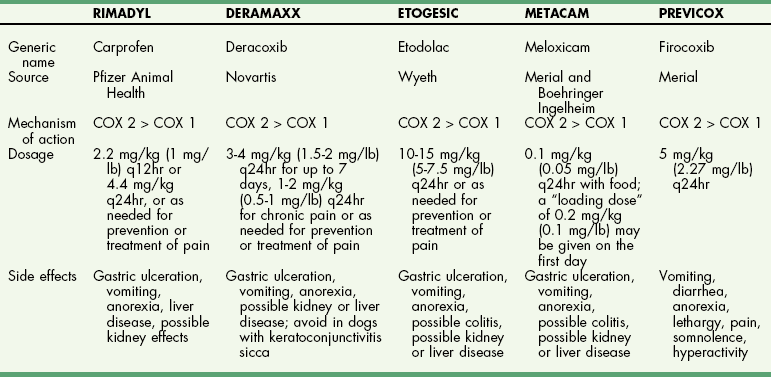
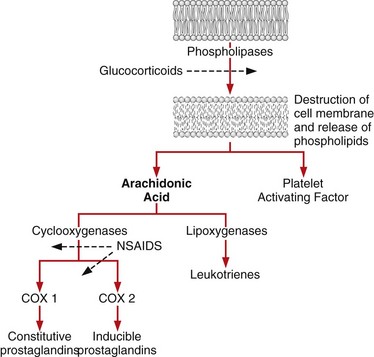
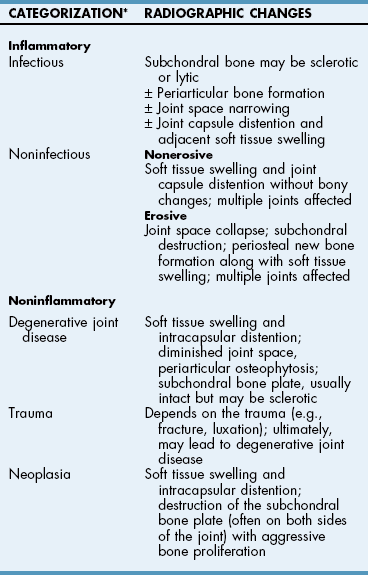
Chapter 34 General Principles and Techniques The common arthropathies of dogs and cats generally are categorized as inflammatory or noninflammatory (Box 34-1). Inflammatory arthropathies are further classified as infectious or noninfectious. Noninfectious arthropathies may be erosive or nonerosive. The common noninflammatory arthropathies in dogs and cats are DJD (which is often due to dysplasia of the hip or elbow or is due to cranial cruciate ligament [CCL] rupture) and those resulting from trauma or neoplasia. Numerous etiologic agents have been associated with infectious arthropathies in dogs and cats, including bacteria, spirochetes (e.g., Borrelia burgdorferi), rickettsiae (Anaplasma phagocytophilum, Ehrlichia ewingii, Neorickettsia risticii, and Rickettsia rickettsii), mycoplasmas, fungi, caliciviruses (cats), bacterial L-forms (cats), and protozoa. Nonerosive, noninfectious arthropathies include idiopathic immune-mediated nonerosive polyarthritis, chronic inflammatory-induced polyarthritis, plasmacytic-lymphocytic synovitis, and arthritis associated with systemic disease (i.e., systemic lupus erythematosus [SLE]). Erosive, or deforming, arthropathies include rheumatoid arthritis, feline chronic progressive polyarthritis, erosive polyarthritis of Greyhounds, and periosteal proliferative arthropathy. The pathophysiologies of the most commonly diagnosed arthropathies are discussed individually in this chapter (see the later discussion). Further information about arthropathies may also be found in most medical texts. Survey radiography is an important and common method of screening affected joints; however, changes with many diseases may be similar, often making radiography nonspecific. Radiographic findings in affected joints include proliferative or erosive bone lesions, increased joint fluid, and adjacent soft tissue changes including muscle atrophy. Radiographic findings help guide clinicians to a definitive diagnosis (Table 34-1); however, absence of radiographic changes does not ensure that the joint is normal. Radiography is particularly insensitive to mild to moderate disease affecting articular cartilage. Radiographic Findings of Arthropathies in Dogs and Cats *Detection of these signs depends on stage of the disease, type of disease, and joint(s) affected. Synovial fluid often is evaluated to help differentiate between arthropathies. Cytologic findings range from normal to the presence of phagocytic mononuclear cells, nondegenerative neutrophils, or degenerative neutrophils plus organisms. Cytologic findings may help make a definitive diagnosis (Table 34-2) or may localize disease, but they are typically nonspecific. Joint taps to obtain synovial fluid are an essential technique for obtaining information to differentiate arthropathies. Sedation or general anesthesia is recommended, especially if the animal is fractious (see p. 1037). Equipment needed includes sterile gloves, 25-gauge needles, 22-gauge Synovial fluid aspirates commonly show no bacterial growth when cultured directly on blood agar plates. More reliable results are obtained if the synovial fluid sample is incubated for 24 hours in a blood culture medium or specific enrichment broth before culturing onto blood agar plates. Select the joint or joints that are swollen for initial taps. Clip the appropriate area over the joint, and prepare the site for an aseptic procedure (Fig. 34-1, A-I). Use a gloved hand to palpate landmarks. Insert a needle attached to a syringe into the joint. Apply gentle suction to the syringe. After the fluid has been collected, release the negative pressure on the syringe and withdraw the needle. If blood appears in the syringe, withdraw the syringe immediately; contamination with blood can alter cell counts. If only a few drops of fluid are obtained (common in small dogs and cats), spray the material directly onto a slide, and examine it cytologically. Estimate viscosity as the fluid drops from the needle to the slide. Normal joint fluid is viscous and forms a long string. Place a drop of fluid on a slide and make a smear for an estimated complete cell count and a differential cell count. Culture fluid for bacterial and mycoplasmal growth: with the same needle and syringe aseptically aspirate an appropriate culture broth (e.g., trypticase soy broth from a blood culture vial). Rinse the barrel of the syringe and hub of the needle with the culture medium, and then inject this culture medium into a blood culture vial with the same medium. Differential diagnoses include all of the noninflammatory and inflammatory arthropathies (see Box 34-1). The definitive diagnosis is made by evaluating the animal’s history, clinical signs, radiographic findings (see Table 34-1), results of other imaging modalities, and joint cytology (see Table 34-2). Medical management of specific arthropathies is provided in the later discussion of specific diseases. Specific therapies may include antibiotics based on culture and susceptibility testing and immunosuppressive medications for immune-mediated joint disease. Regardless of cause, for virtually all joint diseases, there are five basic principles of medical management (Box 34-2). All patients with joint disease except those with additional medical diseases that require specific dietary management can benefit significantly from proper body weight management. Excessive body weight places increased loads on joints, exacerbating concurrent joint disease. Excessive body weight accelerates degeneration of joints with DJD and has been demonstrated to exacerbate clinical signs of osteoarthritis. Excessive body weight also exacerbates signs of dysplastic diseases (e.g., hip dysplasia). Evidence suggests that proper body weight management can lessen and delay clinical signs of osteoarthritis and decrease the need for anti-inflammatory medication and surgery (Huck et al, 2009; Marshall et al, 2010). Recommended body condition scores (BCS) for dogs are 4.5 on a 1 to 9 scale (Table 34-3) and 2.5 on a 1 to 5 scale. Each BCS level above the target level of the 1 to 9 scale represents 10% excess body weight. Weight reduction programs should aim to decrease body weight by 1% to 2% per week until the target BCS is attained. Simplified diet plans recommend feeding 80% to 100% of the caloric resting energy requirements (RER = 70 × [kg of body weight]0.75) for the targeted body weight. When calculating the daily caloric intake, it is critical that all food, including treats, be included. Studies in dogs suggest that omega-3 fatty acids can ease the pain of osteoarthritis. Recent studies have demonstrated that the use of omega-3 fatty acids can improve weight bearing (Roush et al, 2010) and decrease the need for nonsteroidal anti-inflammatory drugs (NSAIDs) in dogs with osteoarthritis (Fritsch et al, 2010). Fatty-acid supplements rarely cause gastrointestinal problems, although sometimes dog owners claim that the supplement has given their dog “fishy-smelling breath.” Omega-3 fatty acids appear to be safe. Alternative therapies for managing osteoarthritis focus on administering chondroprotective agents to slow cartilage degradation and promote cartilage matrix synthesis. Oral chondroprotective supplements are reported to provide supraphysiologic amounts of glucosamine and chondroitin sulfate to the joints to act as precursors for synthesis of hyaline cartilage matrix. These compounds appear to be safe; they cross the gastrointestinal-blood barrier intact when administered orally and may modify pain associated with osteoarthritis. Meta-analysis demonstrates a moderate level of comfort for the efficacy of a combination of glucosamine, chondroitin, and manganese in the treatment of osteoarthritis in dogs (Aragon et al, 2007). Rehabilitation therapy can provide tremendous benefits in the management of small animal joint disease, particularly in dogs (see Chapter 11). The primary targets of physical therapy are strengthening, endurance, and range of motion. Specific recommendations for physical therapy are described in the section on the specific joint diseases. Medical management of DJD often includes therapy with NSAIDs. NSAIDs reduce pro-inflammatory mediators (e.g., thromboxanes, prostaglandins, prostacyclin, and oxygen radicals) by inhibiting cyclooxygenase 1 and 2 (COX-1 and COX-2) pathways. Inhibiting COX-1 inhibits normal physiologic responses in the gastrointestinal and renal systems. Uses of NSAIDs that significantly inhibit COX-1 (e.g., aspirin, ibuprofen, and phenylbutazone) have greater potential to cause gastrointestinal ulceration and/or nephrotoxicity (Fig. 34-2). Most contemporary veterinary NSAIDs (Table 34-4) preferentially inhibit COX-2. It was initially believed that these drugs would provide clinical benefit without the risk of the older NSAIDs; however, the mechanisms of action and adverse effects of these drugs are not as clear as was initially postulated. Numerous studies have reported conflicting results regarding the COX-1:COX-2 ratios of these drugs. However, the method by which an NSAID is determined to affect COX-2 versus COX-1 involves in vitro tests that may or may not accurately reflect what happens in vivo. Presently, it is probably best to say that some drugs appear to be COX-1 sparing instead of COX-2 selective, and it may be more accurate to state that these drugs are “safer” as opposed to saying that they are “safe.” There is a high level of comfort (strong evidence) for the efficacy of NSAIDs in lessening the clinical signs of osteoarthritis in dogs (Sanderson et al, 2009). Finally, some drugs (e.g., tepoxalin) significantly inhibit 5-lipoxygenase, an enzyme that is responsible for leukotriene production (another mediator of inflammation). Side effects of the NSAIDs vary depending on the specific drug and means of reporting adverse events. Owners should be cautioned that anti-inflammatory medication may cause gastrointestinal, liver, or kidney disease (see Table 34-4). Other potential side effects include altered platelet function, extended clotting times, and keratoconjunctivitis sicca (Brainard et al, 2007; Klauss et al, 2007; Luna et al, 2007). Aspirin is commonly used in dogs, but owners should be warned of its propensity to cause gastrointestinal toxicity. Concurrent administration of misoprostol may provide some degree of protection against NSAID-induced gastric lesions (Box 34-3), if needed. Pentosan polysulfate, isolated from beechwood hemicellulose, appears to provide protection against cartilage damage. When given once a week (3 mg/kg SC) it may relieve clinical signs associated with canine chronic osteoarthritis. Although not derived from animal sources, the compound preserves proteoglycan content and stimulates hyaluronic acid synthesis. Pentosan polysulfate may also increase bleeding times. One meta-analysis demonstrated a moderate level of comfort for the efficacy of Pentosan polysulfate in the treatment of osteoarthritis in dogs (Aragon et al, 2007), whereas another demonstrated only weak or no evidence to support efficacy (Sanderson et al, 2009). Polysulfated glycosaminoglycans (PSGAGs) (Adequan) have been routinely used in the management of osteoarthritis in dogs (4 mg/kg IM intramuscular twice a week for 4 weeks). PSGAGs are injectable, highly sulfated glycosaminoglycans marketed for treatment of osteoarthritis. They have a protective effect on cartilage homeostasis in models of osteoarthritis and inconsistently have shown anabolic effects on cartilage metabolism. They have heparin-like activity and may increase bleeding times in dogs. Meta-analysis demonstrated a moderate level of comfort for the efficacy of PSGAGs in the treatment of osteoarthritis in dogs (Aragon et al, 2007). Hyaluronan is a large glycosaminoglycan found in joint fluid and cartilage. In joint fluid, it is a major contributor to viscoelasticity, whereas in cartilage it forms the backbone that links aggrecan molecules. Hyaluronan has been administered intra-articularly to help restore viscosity of joint fluid in arthritic joints. It also has anti-inflammatory activity by interfering with oxygen-free radicals and inhibition of degradative enzymes. There is conflicting information regarding the value of hyaluronan administration in the management of canine osteoarthritis, although there is evidence that it may aid in pain relief of this condition. Two meta-analyses have shown weak or no evidence for the efficacy of hyaluronan in the treatment of arthritis in dogs (Aragon et al, 2007; Sanderson et al, 2009). Antibiotics should be administered for infectious arthritis (see p. 1229) and prophylactically for specific surgical procedures (see Chapter 9). In general, antibiotics should be selected for treatment of bacterial septic arthritis on the basis of identification of the organism and susceptibility testing. Broad-spectrum bactericidal antibiotics should be administered until culture and susceptibility testing results are obtained and then adjusted as necessary (Table 34-5). Gram-positive bacteria are most commonly cultured from septic joints; therefore, initial treatment with first generation cephalosporins is usually indicated before culture and bacterial susceptibility reports are received. If bacterial L-forms are suspected (primarily in cats), doxycycline is the antibiotic of choice. Chloramphenicol and erythromycin might also be effective. If Borrelia spp., Ehrlichia ewingii, Neorickettsia risticii, Anaplasma phagocytophilum, or Rickettsia rickettsii is suspected, doxycycline is the antibiotic of choice. Antibiotics should be administered for 4 to 6 weeks and at least 2 weeks after the cessation of clinical signs. Antibiotics for Septic Arthritis before Culture Results Antibiotics may be administered initially when differentiation between immune-mediated and rickettsial infection has not been made; doxycycline is the antibiotic of choice (see Table 34-5). Doxycycline may be administered until tick titer results are available or as a trial to observe for resolution of clinical signs before initiating steroid therapy for presumptive nonerosive immune-mediated joint disease. Antibiotics should be administered prophylactically to prevent surgical infections, especially in joint replacement procedures, but they are not a substitute for aseptic technique (see Chapters 1, 5, and 6). A broad-spectrum, bactericidal antibiotic, such as cefazolin (22 mg/kg given IV), should be administered after an intravenous (IV) line has been placed and anesthesia induced. This dose can be repeated every 90 minutes to 3 hours during surgery. Antibiotics can be discontinued after surgery, or they may be continued until culture results are obtained. If culture results are negative, antibiotics should be discontinued; if results are positive, antibiotics may be continued or changed according to the results of susceptibility testing. Corticosteroids effectively reduce synovial inflammation by inhibiting activity of phospholipase A (see Fig. 34-2), reducing production of both cyclooxygenases and lipoxygenases. Corticosteroids may also protect cartilage matrix by reducing metalloproteinase activity; however, they depress chondrocyte metabolism and alter the matrix composition by diminishing proteoglycan and collagen synthesis. Because of the adverse systemic effects of long-term corticosteroid administration and their deleterious effects on cartilage, they are seldom indicated for treatment of cartilage injury or DJD. Corticosteroids often are used to treat inflammatory noninfectious arthropathies. When used long term, dosages should be titrated to the smallest amount that achieves an effect, and short-acting steroids (e.g., prednisolone) should be administered every other day if possible to prevent suppression of the hypothalamic-pituitary-adrenal axis; however, inadequate dosage may increase resistance to effective treatment. Efficacy of steroids in the management of immune-mediated joint disease must be based on repeat joint taps and not on clinical evaluation of lameness. Synovial joints permit motion while providing stability for load transfer between bones (Box 34-4). Synovial joint cavities are surrounded by joint capsules made up of an outer layer of fibrous connective tissue lined with a synovial membrane. Nerves, blood vessels, and lymphatic vessels are located between the synovial membranes and the fibrous capsules. Synovial fluid is formed as a dialysate of plasma from the rich vascular supply of synovial membranes. This fluid filters through the vascular endothelium and synovial interstitium to lubricate the joint and provide nutrition for the articular cartilage. The synovial membrane is composed of synovial A and B cells and dendritic cells. Mucoproteins, such as hyaluronic acid, are added to the fluid by synovial B cells; synovial A cells function as phagocytes and secrete interleukin-1 and prostaglandin E. The articulating joint surfaces are covered with 1 to 5 mm of a dense, white connective tissue, usually hyaline cartilage. This articular cartilage facilitates the gliding motion of the joint, distributes mechanical loads, and prevents or minimizes injury to underlying subchondral bone. Some synovial joints (e.g., the stifle joint) also have intra-articular ligaments, menisci, and fat pads that further facilitate joint function and stress reduction during weight bearing (Fig. 34-3). External joint support is provided by surrounding ligaments and tendons. Loss of joint stability occurs after ligament rupture (i.e., ruptured cranial cruciate ligament [CCLR]) or secondary to developmental or anatomic abnormalities (i.e., canine hip dysplasia). Abnormal joint motion places abnormal physiologic loads on portions of the articular cartilage. These loads can cause the cartilage matrix to fracture or fissure, disrupting the collagen fibril network and causing cell death. The tissue response to cartilage loss is similar to that which occurs during healing of cartilage defects (see p. 1225). Subchondral bone sclerosis, osteophyte formation, periarticular soft tissue fibrosis, and synovial membrane inflammation are all definitive signs of DJD, and all are physiologic responses to joint instability. Adult articular cartilage is categorized into four distinct zones based on cellular morphology and spatial arrangement. The superficial zone has a thin, cell-free matrix that provides the gliding surface of articular cartilage. Deep to this layer are thin, elongated chondrocytes oriented parallel to the articular surface. This transitional zone is wider than the superficial zone and is made of spherical chondrocytes and matrix with large collagen fibrils. The deep zone is the largest zone and contains small chondrocytes that are arranged in short columns perpendicular to the joint surface. The deep zone has the highest proteoglycan content and the least water of the cartilage zones. The zone of calcified cartilage is separated from the preceding zones by the “tidemark,” which is visible when articular cartilage is stained with hematoxylin and eosin. The zone of calcified cartilage anchors cartilage to subchondral bone (Fig. 34-4). An arthrotomy is an open surgical approach to a joint using traditional surgical instrumentation. Principles of arthrotomy are listed in Box 34-5. Arthrotomies should be based on a thorough knowledge of the local anatomy, particularly the local musculature and neurovascular structures. Standard approaches to the joints are typically recommended. Make the skin incision in a direction that permits easy retraction of the superficial musculature. When possible, reflect muscles by incising the adjacent fascia versus making an intramuscular incision. If you must incise through the muscles, make your incision parallel to the muscle fibers. Regardless of the technique, be sure that you have adequate exposure to provide proper visualization of the joint. Incise the joint capsule in such a way that it provides exposure while allowing it to be easily closed. At completion of the arthrotomy, close the joint capsule except in diseases in which release of the joint capsule and adjacent fascia may be necessary for proper alignment (i.e., patellar luxation). The joint capsule is not a holding layer; do not close it with tension. Closure of the joint capsule does not need to result in a watertight seal because the joint capsule incision will seal with synovial tissue within days of the surgery. Similarly, if the joint capsule cannot be completely closed, a new capsule will form around the joint rapidly. The fascia surrounding the joint capsule is the holding layer and for most joints plays a role in the stability of the joint. Careful tissue layer apposition is important to joint stability and limb function. Reappose muscles in individual layers to permit proper function, and minimize dead space and seroma formation. Close the remainder of the wound routinely. General principles of arthroscopy are discussed in Chapter 13. Closed reduction of luxated joints is generally preferred to open reduction whenever possible because it minimizes contamination, decreases soft tissue damage, and promotes rapid healing (Table 34-6). When determining if closed reduction and stabilization are appropriate, it is critical to determine if there are underlying diseases (e.g., hip dysplasia, congenital elbow luxation) that significantly decrease the chances of success with closed reduction. Other contraindications to closed reduction include fractures and significant soft tissue interposition. Loss of proteoglycans from matrix occurs with infection, inflammation, and joint immobilization; when cartilage is exposed during surgery; or as a result of traumatic disruption of synovial membranes. With reversible damage, chondrocytes may replace the lost matrix components after the insult is removed. However, irreversible damage may occur. Lacerations or abrasions of the cartilage surface destroy chondrocytes and disrupt the matrix. A standard inflammatory response does not occur with superficial lacerations (those that do not penetrate to subchondral bone) because inflammatory cells from marrow and blood vessels cannot access the joint (Fig. 34-5, A). Chondrocytes near the injury respond by proliferating and synthesizing new matrix; however, this response usually is inadequate for healing. Although superficial lacerations do not heal, they seldom progress. When a full thickness cartilage defect occurs, marrow cells capable of participating in an inflammatory response gain access to the defect (Fig. 34-5, B). The size of the defect affects healing; small defects (1 mm in diameter) heal more completely than large defects. The defect initially is filled with a fibrin clot, which is replaced within 5 days by fibroblast-like cells and collagen fibers. Metaplasia of these fibroblast-like cells into chondrocytes occurs after 2 weeks. These chondrocytes do not function normally, as is indicated by lower concentrations of proteoglycans in the reparative tissue at 6 months after injury. The reparative tissue (fibrocartilage) is also thinner than articular cartilage and is prone to fibrillation and erosive changes. Experimentally, early motion and weight bearing are beneficial for repair of full thickness cartilage defects. Continuous passive motion also appears to accelerate repair of full thickness cartilage defects, which results in repair tissue that more closely approximates hyaline cartilage. Although continuous passive motion is impractical in small animals, physical rehabilitation provides techniques that may have similar benefits. See Chapter 11 for additional information on physical rehabilitation. Physical rehabilitation is an important component of the treatment of joint diseases. Physical rehabilitation increases muscle strength, endurance, and joint range of motion; reduces muscle spasm and pain; and improves performance. Passive physical manipulation, heat, and cold are important considerations in rehabilitation of joint injuries. For additional information on general principles of physical rehabilitation, see Chapter 11. Supervised, regular short exercise periods of walking or swimming, when the animal is no longer showing signs of inflammation or after surgery to stabilize the joint, help strengthen supporting muscles. Exercise should be avoided when the animal shows signs of acute inflammation and should be limited to prevent discomfort. Effects of a physical rehabilitation program can be measured in terms of increases in muscle circumference, joint range of motion documented with a goniometer, and exercise endurance. Aragon, CL, Hofmeister, EH, Budsberg, SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. 2007;230:514. Brainard, BM, Meredith, CP, Callan, MB, et al. Changes in platelet function, hemostasis, and prostaglandin expression after treatment with nonsteroidal anti-inflammatory drugs with various cyclooxygenase selectivities in dogs. Am J Vet Res. 2007;68:251. Fritsch, DA, Allen, TA, Dodd, CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236:535. Huck, JL, Biery, DN, Lawler, DF, et al. A longitudinal study of the influence of lifetime food restriction on development of osteoarthritis in the canine elbow. Vet Surg. 2009;38:129. Klauss, G, Giuliano, EA, Moore, CP, et al. Keratoconjunctivitis sicca associated with administration of etodolac in dogs: 211 cases (1992-2002). J Am Vet Med Assoc. 2007;230:541. Luna, SP, Basílio, AC, Steagall, PV, et al. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res. 2007;68:258. Marshall, WG, Hazewinkel, HA, Mullen, D, et al. The effect of weight loss on lameness in obese dogs with osteoarthritis. Vet Res Commun. 2010;34:241. Roush, JK, Cross, AR, Renberg, WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236:67. Sanderson, RO, Beata, C, Flipo, RM, et al. Systematic review of the management of canine osteoarthritis. Vet Rec. 2009;164:418. Beale, BS. Use of nutraceuticals and chondroprotectants in osteoarthritic dogs and cats. Vet Clin North Am Small Anim Pract. 2004;34:271. Borer, LR, Peel, JE, Seewald, W, et al. Effect of carprofen, etodolac, meloxicam, or butorphanol in dogs with induced acute synovitis. Am J Vet Res. 2003;64:1429. MacWilliams, PS, Friedrichs, KR. Laboratory evaluation and interpretation of synovial fluid. Vet Clin North Am Small Anim Pract. 2003;33:153. This article provides an excellent review of the interpretation of joint fluid. Millis, D, Levine, D, Taylor, R. Canine rehabilitation and physical therapy. Philadelphia: Saunders; 2004. This text contains a complete description of physical therapy for osteoarthritis in dogs. Schulz, KS, Beale, B, Holsworth, et al. The pet lover’s guide to canine arthritis and joint problems. Philadelphia: Saunders; 2005.
Diseases of the Joints
General Considerations
Diagnosis of Joint Diseases
Diagnostic Imaging
![]() TABLE 34-1
TABLE 34-1
Laboratory Findings
Synovial fluid collection.
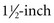 needles (for shoulder, elbow, and stifle joints in larger dogs), 3-inch needles (for the hip joint), and 3-ml syringes. Only a small amount of fluid is needed to determine viscosity, estimated cell count, and differential cell count, and for culture.
needles (for shoulder, elbow, and stifle joints in larger dogs), 3-inch needles (for the hip joint), and 3-ml syringes. Only a small amount of fluid is needed to determine viscosity, estimated cell count, and differential cell count, and for culture.
Differential Diagnosis
Medical Management
Principle 1: Weight Management
Principle 2: Nutritional Supplementation
Slow-acting, disease-modifying osteoarthritis agents.
Principle 4: Physical Rehabilitation Therapy
Principle 5: Nonsteroidal Anti-inflammatory Drug Therapy and Other Medical Therapies
Other medical therapies.
Antibiotics.
![]() TABLE 34-5
TABLE 34-5
SUSPECTED TYPE
ANTIBIOTIC
Gram-positive
Cephalexin 22 mg/kg PO, IV q8-12hr
Amoxicillin-clavulanate 12.5-25 mg/kg PO q12hr
Borrelia, rickettsiae, Mycoplasma, bacterial L-forms
Doxycycline 5 mg/kg PO q12hr
Gram-negative
Baytril 5-20 mg/kg PO, IV* q24hr
Anaerobes
Metronidazole 10 mg/kg PO, IV* q12hr
Corticosteroids.
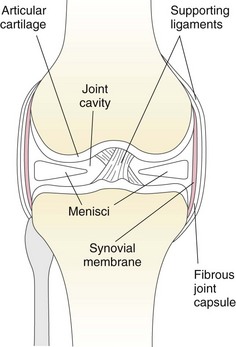
Surgical Anatomy
Surgical Technique
Endoscopically Assisted Joint Surgery
Closed versus Open Joint Reduction
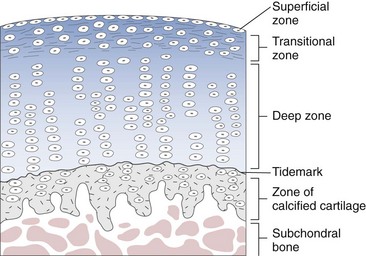
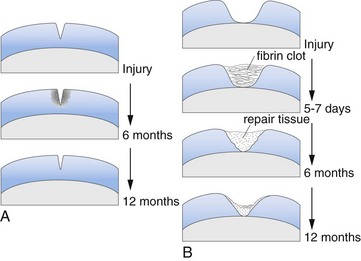
Healing of Cartilage Defects and Response of Cartilage to Treatment
Continuous Passive Motion
Postoperative Care and Assessment
Physical Rehabilitation
References
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diseases of the Joints