Chapter 4 Surgery of the orbit
Surgical management of orbital diseases 51
Ancillary diagnostic procedures 52
Surgical anatomy of animal orbits 53
TYPES OF ORBITAL SURGICAL PROCEDURES 60
Enucleation procedures in small animals 60
Evisceration with intraocular prosthesis in small animals 63
Exenteration in small animals 67
Enucleation procedures in large animals and special species 67
Orbitotomy in small animals 76
Total and partial orbitectomy 81
Orbitotomy in large animals and special species 81
Surgical management of traumatic proptosis in small animals 82
Surgical augmentation of orbital volume in small animals 83
POSTOPERATIVE CARE AND MANAGEMENT 84
Postoperative complications and treatment in all species 84
Surgical management of orbital diseases
Ancillary diagnostic procedures
Surgical anatomy of animal orbits
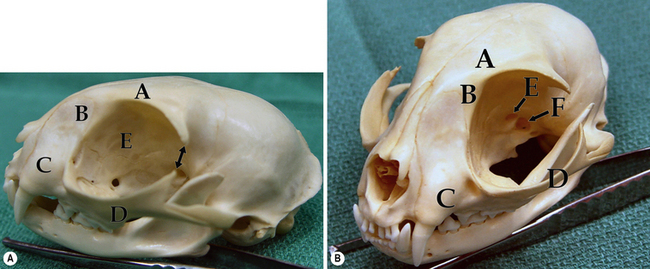
Canine orbit
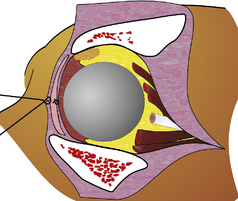
The bones that envelop and confine the orbital tissues in the dog consist of the zygomatic process of the frontal bone dorsally; the frontal bone and palatine bones medially; and the zygomatic arch and vertical ramus of the mandible laterally (Fig. 4.1a). The bony orbital floor is comprised of the sphenoid bone. The orbital rim consists of the zygomatic process of the frontal bone dorsally, the lateral orbital ligament, and parts of the zygomatic, maxillary, and lacrimal bones ventrally. Soft tissues that support the orbital walls include the temporalis muscles posteromedially, the temporalis and pterygoid muscles medially, the masseter muscle laterally, and the medial pterygoid muscle ventrally. The zygomatic or orbital salivary gland is located in the dog in the rostrolateral orbit. In brachycephalic breeds of dogs the orbit is very shallow, while in dolichocephalic breeds the orbit is considerably deeper and difficult to access surgically.
The third (oculomotor), fourth (trochlear), and sixth (abducens) cranial nerves innervate the retrobulbar muscles, and the second (optic), fifth (trigeminal), and branches of the seventh (facial) cranial nerves permit vision, sensation, and lacrimation (Fig. 4.1b). The orbit also contains autonomic fibers, with the sympathetic fibers extending from the superior cervical ganglion, and parasympathetic fibers entering the orbit to synapse in the ciliary ganglion. Parasympathetic fibers from the ciliary ganglion then continue to innervate the iris sphincter and ciliary body muscles.
Feline orbit
The orbit of the cat is similar but not identical to the dog. In contrast to the dog, the feline orbit is only slightly larger than the globe, which greatly restricts orbital exploration unless the globe is first removed. The bones that compose the orbital walls include the sphenoid, maxillary, lacrimal, zygomatic, and frontal (Fig. 4.2). The lateral orbital ligament joins the frontal and zygomatic processes. The bony floor of the feline orbit consists of only a small shelf of maxillary bone that holds the last molar teeth. The extraocular muscles are small and ocular mobility is limited. The zygomatic or intraorbital salivary gland is small in the cat and lies close to the maxillary nerve. The cat’s orbital measurements are about 87 mm long, 26 mm wide, and 23 mm high. The feline globe ranges in size from 20 to 22 mm anteroposteriorly, 19 to 20.7 mm vertically and 18 to 21 mm transversely. The larger Siamese breed globes measure 22.5 mm anteroposteriorly and 22.5 mm transversely.
Horse orbit
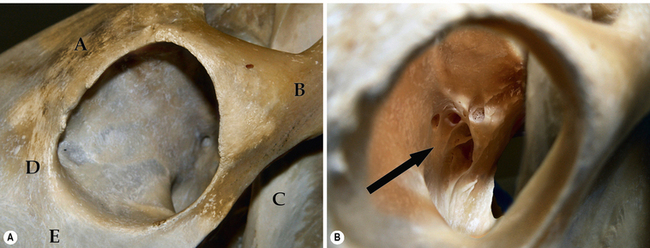
Both the horse and cow orbits are among the largest that clinically confront the veterinarian. The orbital bones in the horse include the frontal, lacrimal, zygomatic, temporal, sphenoid, palatine and maxillary (Fig. 4.3a). The bones contributing to the equine orbital rim include the lacrimal (ventromedial orbital rim), the frontal and its zygomatic process (dorsal orbital rim), and the zygomatic processes of the temporal and zygomatic bones (incomplete lateral wall and lateral canthus). The zygomatic process of the frontal bone contains the supraorbital foramen, an important landmark for supraorbital nerve blocks to produce upper eyelid regional anesthesia and paralysis. Hence, the entire orbital rim in horses consists of bones and no fascial tissues or ligaments. The lacrimal bone contains both a shallow fossa for the poorly developed lacrimal sac as well as the entry of the nasolacrimal system into the nasal turbinates.
Four important foramina are sited in the apex of the orbit (Fig. 4.3b). They include:
1. the ethmoid foramen – entry for ethmoid blood vessels and nerves
2. the optic foramen – exit of the optic nerve
3. the orbital fissure – transmits the ophthalmic branch of the trigeminal nerve, the oculomotor (third) nerve, the abducens (sixth) nerve, and often the trochlear (fourth) nerve
4. the round foramen – maxillary branch of the trigeminal nerve.
Cow orbit
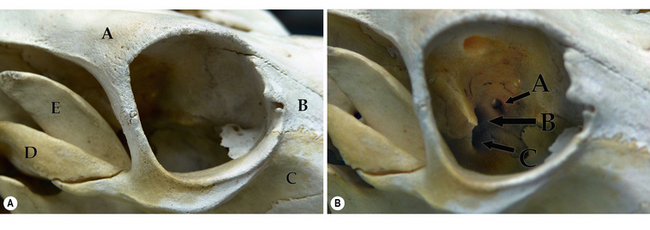
The cow orbit has many similarities to the horse, but also significant differences. For instance, the frontal bone is very large and well developed to accommodate the cow’s horns. The bones which contribute to the bovine orbit include the frontal, lacrimal, zygomatic, palatine, maxillary, and sphenoid. The bovine orbital rim, composed of three bony structures around 360°, consists of: 1) the frontal bone (dorsal rim); 2) the lacrimal bone (medial canthus); and 3) the zygomatic bone and frontal process of the zygomatic bone (entire ventral rim and lateral canthus). The coronoid process of the mandible is well developed and positioned just caudal to the lateral rim to permit hypodermic needle insertion into the deep orbital tissues from behind the lateral orbital rim (Fig. 4.4a). The bony orbital walls consist of: 1) the lacrimal and sphenoid bones medially; 2) the palatine and sphenoid bones ventrally; 3) the frontal bone dorsally, and 4) the temporal and zygomatic bones laterally.
Important orbital foramina include: 1) the ethmoidal foramen (ethmoidal blood vessels and nerves); 2) the optic foramen (passage of the optic nerve); and 3) the orbitorotundum (a combination of the orbital fissure and the foramen rotundum) for passage of the oculomotor (third), the trochlear (fourth), the trigeminal (fifth) and the abducens (sixth) nerves, and retinal and maxillary blood vessels (Fig. 4.4b).
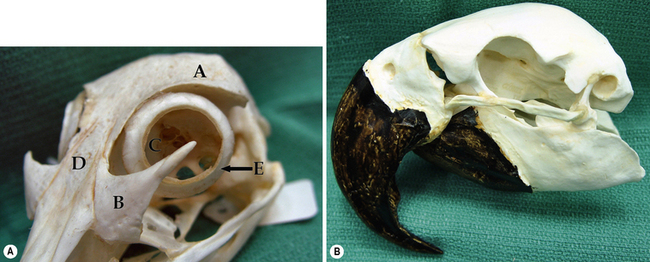
Avian orbit
Avian species that are presented to veterinarians for eye disease are generally in the raptor group (owls, falcons, and hawks) and the psittacines (parrots, cockatiels, and parakeets). Trauma in the raptor group is probably the most frequent single cause of eye disease in this group, and the most treatable. Orbital anatomy varies markedly in the avian species, based on the shape of the skull and beak (Fig. 4.5).
Surgical pathophysiology
Orbital inflammation: acute and chronic
The animal orbit is susceptible to bacterial infections (Fig. 4.6). Orbital cellulitis may present as acute or chronic, and is usually associated with bacterial or fungal infections (often entry cannot be ascertained), as well as foreign bodies. Orbital cellulitis occurs most frequently in dogs (especially the hunting breeds), and is rare in cats. In horses, cattle, and certain species of birds orbital cellulitis may be secondary to adjacent sinus infections or as a sequel of de-horning in cattle. Fungal infections are infrequent in the dog, and are usually associated with foreign bodies. Infection may enter the orbit through several routes. Infectious agents can enter from the mouth, conjunctivae, the adjacent sinuses and nasal cavity, the subcutaneous and skin surfaces of the incomplete lateral and dorsolateral orbital walls, and hematogenously. In a recent report on orbital abscesses in dogs and cats, the most common bacterial genera isolated from dogs were Staphylococcus, Escherichia, Bacteroides, Clostridium, and Pasteurella. The most frequent bacteria isolated from orbital abscesses in cats were Pasteurella and Bacteroides. The highly vascular orbit and the endorbita that covers the orbital tissues usually respond quickly to antibiotic therapy. This orbital compartmentalization can also impede the spread of the infectious nidus, but also foster the development of focal septic areas that impede antibiotic penetration. As a result, surgical excision of chronic orbital abscesses and focal granulomas may be necessary for complete resolution of the condition.
Orbital neoplasms
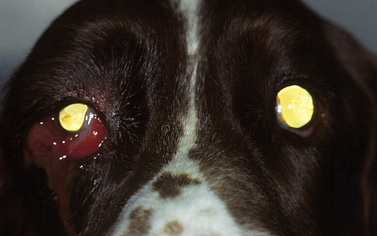
Orbital neoplasms are not infrequent in dogs, but are less common in cats. In both horses and cattle, intraorbital lymphomas, lymphosarcomas, and squamous cell carcinomas are the most frequent types. In dogs, orbital neoplasms consist of a large number of different tumor types, while in cats the most frequent orbital neoplasm is squamous cell carcinoma. Primary orbital neoplasms can arise from any tissue (epithelial, vascular, neural, and connective tissues) within the orbit. Secondary orbital neoplasms also occur and invade locally from the nasal, sinus, and cranial cavities, as well as metastasize from distant sites. The clinical signs of orbital neoplasia are usually associated with a slowly enlarging and painless mass within the orbit (Fig. 4.7). Depending on its position, a neoplasm within the orbit can produce strabismus; the direction of the ocular deviation may assist to localize the mass.
Perioperative medications
For lateral and dorsal orbitotomy procedures, standard skin preparation is recommended. The planned surgical site is clipped, and cleaned with surgical antimicrobial soap. The area is wiped with iodine (0.5% dilution) and alcohol, and carefully draped, leaving the surgical area exposed. For enucleation and other surgical procedures performed through the palpebral fissure, the eyelids, corneal and conjunctival surfaces are prepared for surgery as outlined in Chapter 2.
Types of orbital surgical procedures
Enucleation procedures in small animals
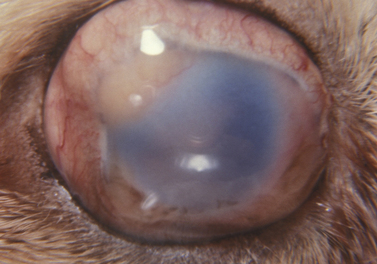
In enucleation, the globe and its contents are excised. In animals, the indications for enucleation include: 1) ocular congenital defects, such as microphthalmia, that result in chronic problems such as conjunctivitis and keratitis; 2) intraocular infections that have destroyed the globe, and are potential sources of systemic infection; 3) intraocular tumors not amenable to local excision or laser therapy and still confined to the globe (Fig. 4.8); 4) proptosis of the globe with several of the extraocular muscles and/or the optic nerve severed; 5) intraocular inflammation that has destroyed the intraocular tissues and resulted in blindness; and 6) extensive trauma to the globe with the loss of intraocular tissues and without the possibility of successful repair.
Subconjunctival enucleation
In the subconjunctival procedure for enucleation, entry into the orbit is through the bulbar conjunctiva. After completion of draping around the palpebral fissure, a 5–10 mm lateral canthotomy may be performed to increase exposure (Fig. 4.9a). With blunt-tipped tenotomy, strabismus, or Metzenbaum scissors, the full-thickness lateral canthus is cut. Hemostasis is usually achieved by direct pressure with a surgical sponge, if necessary supplemented by point electrocautery. The bulbar conjunctiva and Tenon’s capsule are incised at the 12 o’clock position by curved Steven’s tenotomy, strabismus, or Metzenbaum scissors with blunt tips for about 3–5 mm posterior to the limbus, and the incision extended for 360° (Fig. 4.9b). Using the scissors’ blunt tips, the dissection plane between the sclera and Tenon’s capsule is extended deeper into the orbit until each extraocular muscle insertion is identified (Fig. 4.9c). After isolation with a muscle hook, the tendinous insertions of all of the extraocular muscles are incised. Transection of the extraocular muscle insertions, rather than through the muscle per se, minimizes hemorrhage. As each of the four major rectus muscle insertions is incised, the globe becomes more mobile. After incision of the retractor muscle and oblique muscle insertions, the globe will displace slightly forward.
To sever the optic nerve and the adjacent posterior ciliary arteries, a small curved hemostat or enucleation forceps are carefully positioned posterior to the globe (Fig. 4.9d). With curved Metzenbaum scissors or the specially curved enucleation scissors, the optic nerve and surrounding blood vessels are transected just anterior to the hemostat. Placement of the scissors is critical to avoid any contact with the posterior sclera and to prevent inadvertent incision of the posterior segment of the eye. The globe is carefully removed from the orbit to permit placement of a ligature deep to the hemostat still clamped to the optic nerve and accompanying blood vessels. The orbit is now carefully examined for any bleeders, and ligatures or point electrocautery applied if needed.
If an intraorbital implant is not used, parts of the remaining extraocular muscles and periorbital fascia are apposed with 2-0 to 4-0 simple interrupted absorbable sutures to reduce the dead space within the orbit. The remaining bulbar conjunctiva and anterior Tenon’s capsule are apposed with 2-0 to 4-0 simple interrupted absorbable sutures. With closure of the bulbar conjunctiva, 4–6 mm of the eyelid margins (including the medial and lateral canthi, and nictitating membrane) are excised circumferentially with tenotomy or strabismus scissors. The nictitating membrane is protracted, and two hemostats are overlapped and clamped at its base (Fig. 4.9e). The remaining nictitating membrane, complete with gland, is excised by tenotomy or strabismus scissors. The remaining eyelids (including the septum orbitale) are closed and apposed with 3-0 to 5-0 simple interrupted non-absorbable sutures (Fig. 4.9f,g).
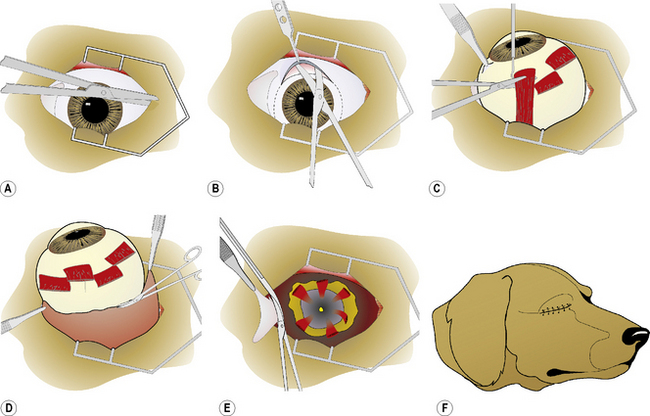
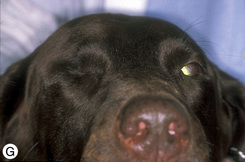
If an orbital prosthesis is planned, an 18–22 mm sterile silicone sphere (Jardon Eye Prosthetics Inc., Southfield, MI) or methyl methacrylate sphere (Storz Instrument Company, St Louis, MO) is usually selected (Fig. 4.10). The surface of the silicone sphere is scarified or roughened with several incisions via scalpel blade to roughen its smooth surface and facilitate orbital retention. The sphere is inserted, and the extraocular muscles and endorbita are apposed about the sphere. An alternative method is the placement of mesh implants on the anterior surface of the orbital rim to prevent postoperative eyelid and orbital shrinkage.
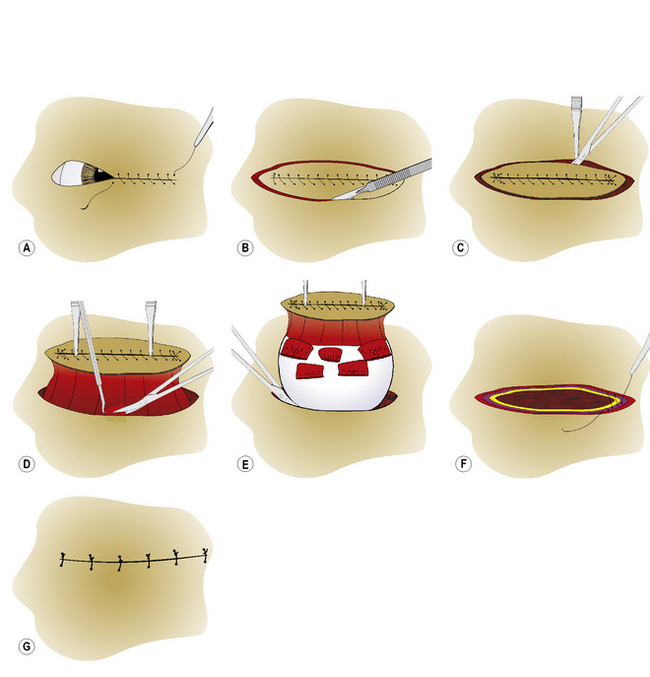
Transpalpebral (‘en bloc’) enucleation
After draping, the eyelids are apposed with simple continuous 3-0 to 4-0 sutures, thereby closing the palpebral fissure (Fig. 4.11a). The eyelid skin is incised circumferentially by scalpel blade about 6–8 mm from the eyelid margins to avoid the bases of the meibomian or tarsal glands (Fig. 4.11b). The skin incision is carefully deepened until the submucosa of the palpebral conjunctiva is reached. Then, with blunt dissection with Steven’s tenotomy, strabismus or Metzenbaum scissors, the incision is continued under the conjunctival fornices, and onto the globe and under the bulbar conjunctiva (Fig. 4.11c). The procedure continues using the same steps as the subconjunctival method. Dissection within the sub-Tenon’s space between the sclera and Tenon’s capsule will usually minimize hemorrhage. All of the extraocular muscles are severed at their insertions (Fig. 4.11d). Isolation, clamping by curved hemostat, incision of the optic nerve, and removal of the globe follow (Fig. 4.11e). The Vicryl® ligature is carefully positioned deep to the hemostat on the optic nerve stump.
Closure of the anterior periorbital fascial tissues with simple interrupted 3-0 to 5-0 absorbable sutures, with or without an orbital prosthesis, helps to reduce the dead space within the orbit. The orbital septum within the eyelids is apposed with 3-0 to 4-0 simple interrupted or horizontal mattress absorbable sutures (Fig. 4.11f). The eyelid–subcutaneous layer is apposed using the same type of suture and suture pattern. The eyelid skin is apposed with several 3-0 to 4-0 simple interrupted non-absorbable sutures (Fig. 4.11g).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree