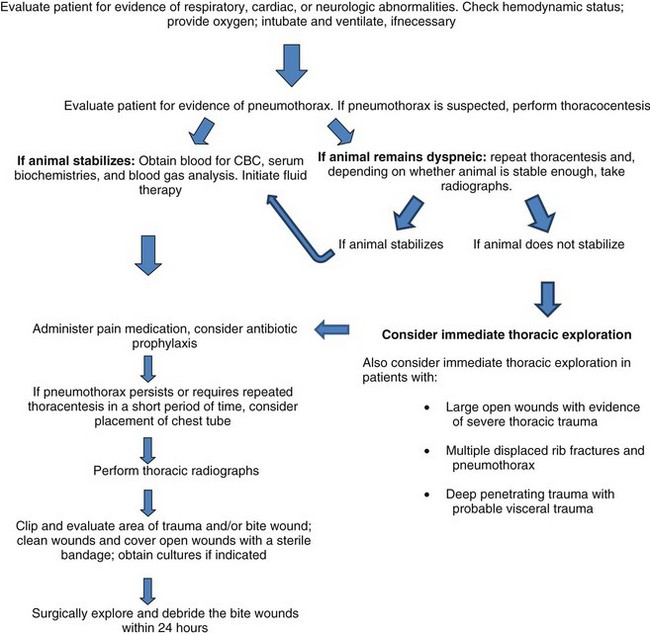
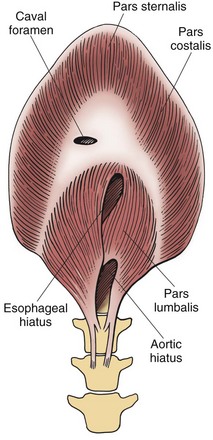
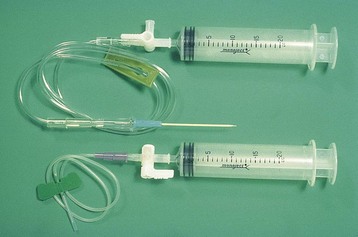
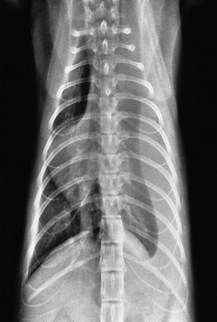
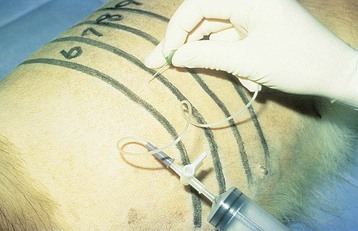
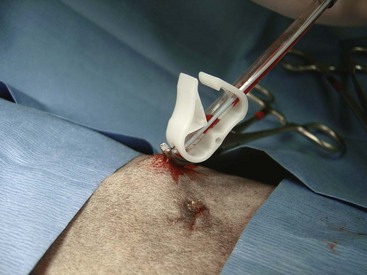
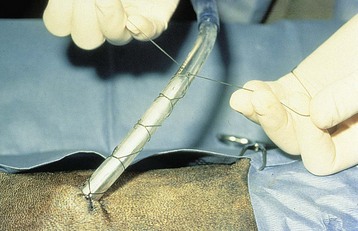
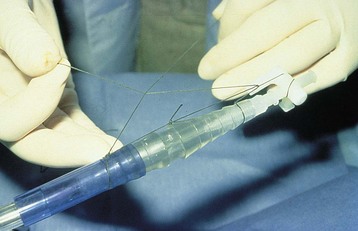
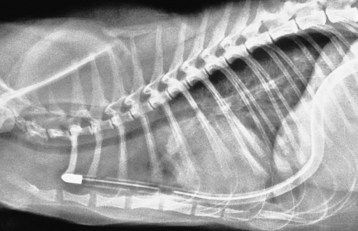
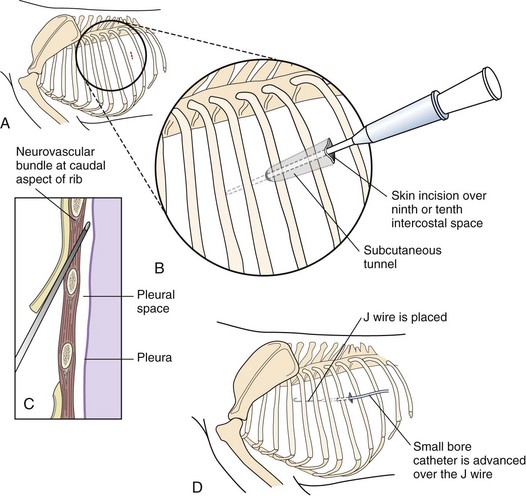
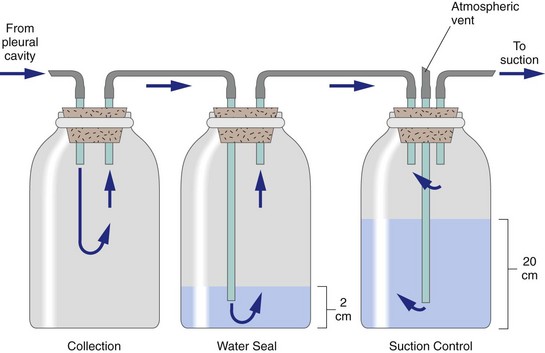
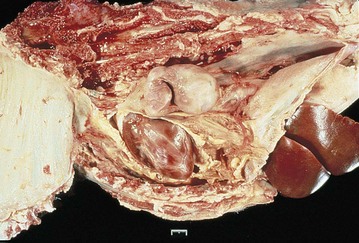
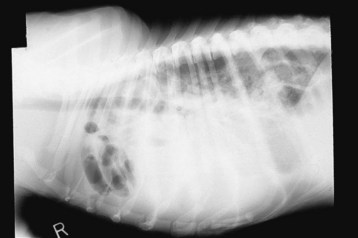
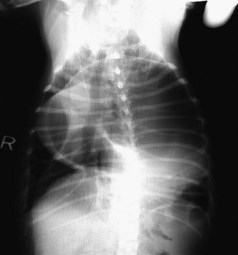
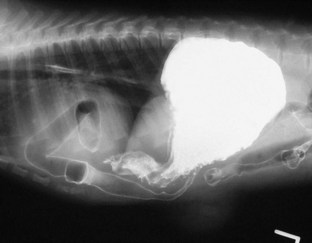
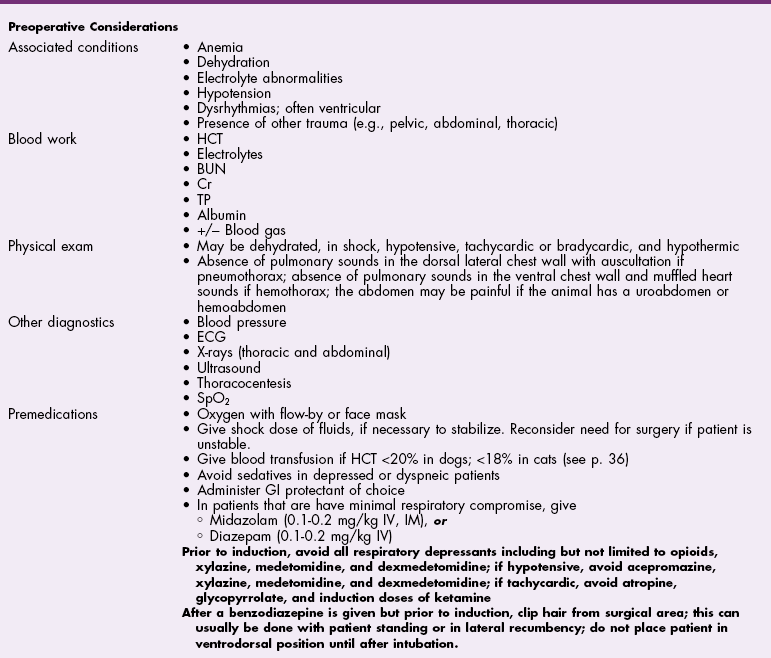
Chapter 31 General Principles and Techniques Respiratory function should be carefully monitored in patients with pleural cavity or diaphragmatic abnormalities. Qualitative assessments of respiratory function include monitoring the respiratory rate and pattern plus capillary refill time and color (Table 31-1 and Box 31-1). Animals with pleural cavity disease usually have a restrictive respiratory pattern (i.e., rapid, shallow respirations). Arterial blood gas analysis can augment qualitative information about the effectiveness of ventilation and gas exchange (Table 31-2). Pulse oximetry noninvasively measures hemoglobin saturation of blood and thus indirectly provides quantitative information about oxygenation. Cardiovascular parameters (i.e., heart rate and rhythm) should also be evaluated (see Table 31-1). An electrocardiogram (ECG) should be performed in all trauma patients to detect arrhythmias resulting from traumatic myocarditis. Intravenous fluids should be provided to dehydrated animals or those drinking insufficient fluids to maintain hydration. Care should be taken to avoid causing overhydration and pulmonary edema, which further compromise respiration. Monitoring central venous pressure may be useful in some patients. Normal Heart Rate (HR) and Respiratory Rate (RR) in Conscious Dogs and Cats Normal pH and Arterial Blood Gas Values on Room Air Severely dyspneic animals strongly suspected of having pneumothorax or pleural effusion should have thoracentesis (Fig. 31-1) before radiographs are made. If the patient is not severely dyspneic, it is safer to first obtain a dorsoventral thoracic radiograph lest lung laceration during thoracocentesis cause pneumothorax and worsen the dyspnea. Removal of even small amounts of pleural effusion or air may significantly improve ventilation, allowing safer manipulation of the patient for radiographic procedures. Most dyspneic animals allow thoracentesis with minimal restraint; general anesthetics are contraindicated. The animal should be allowed to remain in sternal recumbency, and oxygen should be provided by a face mask, flow-by, or nasal insufflation if the animal will tolerate it (see Chapter 4). A negative tap does not rule out pleural effusion or pneumothorax (i.e., the fluid may be in pockets, there may be exudate plugging the needle, or the needle may be too short). If there is no evidence of third space disease or if removal of fluid or air from the chest does not help alleviate dyspnea, then underlying pulmonary disease (i.e., pneumonia, pulmonary edema, pulmonary contusions, pulmonary neoplasia) or severe fibrosing pleuritis should be suspected (discussed later). Providing nasal oxygen or placing the animal in an oxygen cage may be beneficial while treatment of the pulmonary disease is initiated (see p. 31). Chest tube placement should not be attempted in animals with severe respiratory distress. Generally, stabilization and improved ventilation can first be accomplished by removing some pleural air or fluid by means of needle thoracentesis. In critically ill patients, large bore or trocar chest tubes occasionally can be placed without the use of general anesthesia; local anesthesia (i.e., local anesthetic infiltration or an intercostal nerve block) may be sufficient (Box 31-2). However, this should be avoided if at all possible because animals with pleural cavity disease benefit from intermittent positive pressure ventilation and oxygen supplementation during tube placement. Control of the animal’s airway (by means of endotracheal intubation and positive pressure ventilation) and oxygen therapy should be achieved rapidly (discussed later). The use of small-bore (e.g., 14-gauge) wire-guided chest drains (Chest Drain; MILA International, Inc.) placed using a modified Seldinger technique has recently been reported in animals (Valtolina and Adamantos, 2009). These catheters may be placed in some animals with sedation only. For preoperative concerns of patients with diaphragmatic herniation, see page 1004. The cross-sectional anatomy of the thorax in clinically normal dogs (DeRycke et al, 2005) and cats has been compared to findings obtained on computed tomography (CT) and may be useful for surgeons as a preoperative resource. Most structures of the thorax can be visualized on CT images at the soft tissue setting. CT allows for 3D reconstruction and elimination of the superimposition of overlying structures, thus permitting a more precise characterization of abnormalities prior to surgical intervention than is obtained radiographically. For example, tracheobronchial lymph node evaluation via CT has been compared in dogs with known tracheobronchial lymph node histopathology and in clinically normal dogs (Bellegeer et al, 2010). Absolute lymph node size and anatomically normalized lymph node ratios were significantly correlated with metastasis or severe granulomatous lymphadenitis, and lymph node contrast enhancement pattern was also significantly correlated to disease in the aforementioned study. Respiratory patients should be managed with extreme care until they have been intubated and ventilation can be assisted. Premedication with any drug that causes hypoventilation is contraindicated (Table 31-3). Additionally, every attempt should be made to minimize stress to the patient prior to induction. Oxygen support, even while placing an intravenous catheter, may be necessary. Pulse oximetry with a sensor that can be secured to the tail can be especially helpful with perioperative monitoring of the respiratory distressed patient. Prior to induction, the anesthesia provider should be prepared with airway devices, anesthesia machine, and monitors, as well as induction and emergency drugs. Preoxygenating the patient for 3 to 5 minutes prior to induction (see p. 992) should be followed with a rapid induction and intubation. If possible, positive pressure ventilation should be performed with lower tidal volumes, lower peak ventilation pressures, and higher respiratory rates. This will decrease leakage of air through damaged alveoli if pneumothorax is present. On the other hand, if the diagnosis is diaphragmatic hernia or pleural effusion, tidal volumes may be kept low, but peak airway pressures may need to be higher to accomplish adequate ventilation. If external pressures asserted on the lung tissue are completely compressing alveoli, bronchioles, and bronchi, it may take higher pressures to ventilate until the thorax is surgically opened. When possible, however, smaller tidal volumes, higher respiratory rates, and lower peak airway pressures should be used. Dogs and cats with respiratory insufficiency should be maintained with inhalation anesthetics (i.e., isoflurane or sevoflurane) (see Table 31-3). Inhalation anesthesia is advantageous because it allows rapid recovery and more precise control of anesthetic depth than does maintaining anesthesia with longer-acting intravenous anesthetics Nitrous oxide should not be used in patients with pneumothorax or diaphragmatic hernias because it rapidly diffuses into air-filled spaces (i.e., pleural cavity or gas-filled organs), causing further lung compression or organ enlargement. Also, nitrous oxide is comparatively less soluble in plasma than are oxygen and other inhalation anesthetics; therefore, it rapidly diffuses into the alveoli when it is discontinued, resulting in diffusion hypoxia if the nitrous oxide is not turned off 5 to 10 minutes prior to extubation. Once the surgery has been completed, extubation should not be rushed. Make sure the patient is awake and not overly sedated, respirations are adequate, and the patient is comfortable prior to extubation. For specific anesthetic recommendations for animals with pneumothorax and diaphragmatic hernias, see pages 1004 and 1013, respectively. See also Chapter 30. Anesthetic Considerations for Thoracotomy of the Acute Trauma Patient *Monitor for hyperthermia in cats. Needle thoracentesis performed with proper aseptic technique is unlikely to induce infection in patients with normal immune function; therefore, prophylactic antibiotics are not indicated. Prophylactic antibiotics in patients with chest tubes are controversial. Studies in human beings have not shown lower infection rates when patients with chest tubes are given prophylactic antibiotics. However, chest tubes must be maintained and handled with appropriate precautions (e.g., sterile gloves and syringes, chest bandages) to reduce the potential for iatrogenic contamination. Gram-negative bacteria and anaerobes are common isolates in animals with respiratory disease. Therapy of pyothorax should be based on culture and sensitivity test results, if possible, because unpredictable antibiotic sensitivity is common with the microorganisms commonly encountered with this condition. For specific antibiotic recommendations for patients with pyothorax, see page 1027. Fibers of the diaphragm arise from attachments on the ventral surface of the lumbar vertebrae, ribs, and sternum and radiate toward the tendinous center (Fig. 31-2). The diaphragm is composed of a central tendinous portion and an outer muscular portion. The costal part of the diaphragm attaches to the internal surface of the last few ribs, and the central portion extends cranially into the thoracic cavity. Treatment of pleural cavity disease varies, depending on the underlying etiology. For traumatic pneumothorax (see p. 1013), intermittent needle thoracentesis may be sufficient in some animals to prevent dyspnea while the lung heals, but chest tubes occasionally are required. With some types of pleural effusion (i.e., pyothorax; see p. 1027), tube thoracentesis and thoracic lavage are mandatory in the primary treatment of most affected animals. Needle thoracentesis is performed with a small-gauge butterfly needle (No. 19 to No. 23) attached to a three-way stopcock and syringe, or an over-the-needle catheter attached to an extension tubing, three-way stopcock, and syringe (Fig. 31-3). Be sure the needle is long enough to penetrate to the pleural space in large or obese animals. In such animals, a catheter rather than a butterfly needle may be needed. The appropriate site for thoracentesis should be selected based on the physical examination or, if available, radiographs or ultrasound. The mediastinum in dogs and cats is thin and permeable to fluids, and aspiration of one side of the thorax typically drains the contralateral hemithorax adequately. With some diseases, particularly chylothorax and pyothorax, unilateral effusions may occur as a result of thickening of the mediastinum associated with chronic inflammation (Fig. 31-4). Unless there is a reason to go elsewhere, perform thoracentesis at the sixth, seventh, or eighth intercostal space, near the level of the costochondral junction (Fig. 31-5). Clip the selected site and perform a local anesthetic block if needed (this is rarely the case). Aseptically prepare the site, and introduce the needle into the middle of the selected intercostal space. Carefully avoid large vessels associated with the posterior aspect of the rib margins. Advance the needle into the pleural space. Aspirate fluid while the needle is being advanced to allow prompt recognition of the appropriate depth of needle placement. If you feel the heart beating or lungs rubbing across the tip of the needle, withdraw the needle and reassess the situation. With the bevel of the needle facing inward, orient the needle against the rib cage to prevent damage to the lung surface. Gently aspirate fluid and place 5-ml samples in an ethylenediamine tetra-acetic acid (EDTA) tube and a clot tube for a cell count and biochemical parameters, respectively. Also, make six to eight direct smears for cytologic evaluation. Submit samples for aerobic and anaerobic cultures. Equipment needed for a tube thoracostomy include a chest tube, an apparatus to connect the tube to a syringe or to a continuous suction bottle, and a device to collect the drained material (syringe or collecting bottle). Large bore commercially available tubes usually are made of polyvinyl chloride or silicone rubber and are less reactive than red rubber feeding tubes. Commercial tubes come with a metal stylet that simplifies tube placement but may increase the risk of perforating lung tissue compared with red rubber feeding tubes. The red rubber feeding tubes usually are inserted using a large hemostat or Carmalt clamp. Commercial chest tubes come in various sizes ranging from 14 to 40 French. Both large and small bore chest tubes have been advocated in the veterinary literature. When using large bore tubes, the size of the thoracostomy tube should generally approximate the diameter of the mainstem bronchus; smaller tubes may be adequate for removing air, whereas larger tubes may be required with more viscous effusions (Box 31-3). If a commercial tube is used, attach it by means of a tube adaptor (e.g., five-in-one connector, Christmas tree adapter) to either a three-way stopcock or the tubing from a continuous suction device. The ends of red rubber feeding tubes can be cut to accommodate a three-way stopcock; attaching these tubes to a continuous suction device generally is not recommended because of their tendency to collapse (Box 31-4). The use of small-bore wire-guided chest drains has been reported in veterinary patients (Valtolina and Adamnatos, 2009). Advantages of these tubes over larger tubes may be fewer insertional and infectious complications when compared to larger tubes; however, large veterinary studies evaluating complications with these tubes have not been reported. Potential complications of chest tube placement (large or small bore) include pneumothorax, kinking or malpositioning of the tube, pyothorax, hemorrhage from intercostal vessels, leakage around the catheter, and damage to or premature removal of the tube by the animal. Pneumothorax, kinking, and malpositioning are the most commonly reported complications associated with the use of small-bore catheters (Valtolina and Adamnatos, 2009). A subcutaneous vascular access port (VAP) attached to intrathoracic drain tubing has been reported to be effective for removing pleural effusion in dogs where chronic removal (i.e., months to years) was likely to be necessary (Cahalane et al, 2007). The VAP may be attached to regular chest tube or to a Jackson-Pratt drain tube (Jackson-Pratt, Cardinal Health, McGaw Park, Illinois) (Fig. 31-6). FIG 31-6 Illustration depicting placement of a VAP and Jackson-Pratt drain for treatment of pleural effusion in a dog (views from the left side). Notice the cervical subcutaneous location of therapy and the intrathoracic location of the Jackson-Pratt drain. (Reprinted with permission of author and publisher. Calhane et al: J Am Vet Med Assoc 230:527, 2007.) Clip and prepare the lateral thorax for aseptic surgery. To allow sufficient drainage, place additional holes in the tube by bending the tube and removing a notch with a pair of sterile scissors (Fig. 31-7). Make sure that the holes are no larger than one third the circumference of the tube. Be certain that all the holes will easily fit within the thoracic cavity. If using a commercial tube with a radiopaque line, place the last hole through the line to allow identification of its position on a thoracic radiograph. Make a small skin incision in the dorsal one third of the lateral thoracic wall at the level of the tenth or eleventh intercostal space. Advance the tube subcutaneously in a cranioventral direction for three to four intercostal spaces, and introduce the tube through the muscle and pleura using the stylet or a large hemostat. When using a trocar tube, firmly grasp the tube 1 to 2 cm from the body wall with one hand while using the other hand to “pop” the tube through the intercostal musculature and pleura (Fig. 31-8). This prevents the tube from being inadvertently pushed farther into the thorax than anticipated, thereby damaging the lung or other thoracic structures. Feed the tube in a cranioventral direction to a predetermined point; before completely removing the trocar, clamp the tube with a hemostat. For added safety when the chest cavity is not being suctioned, clamp the tube where it exits the body wall with a hemostat or tube clamp device (Fig. 31-9). The latter device is preferred as it will not damage the tube and is more comfortable for the patient than a hemostat. Place a purse-string suture in the skin around the tube (do not enter the lumen of the tube), and leave both ends of the suture long. Use this suture to make a Chinese finger-trap or Roman sandal suture (Fig. 31-10). Connect the chest tube to a three-way stopcock to increase the ease of thoracic drainage. Use a tube adapter or female Luer-Lok (with small tubes) between the tube and the three-way stopcock to ensure an airtight seal. Use suture to secure the tube to the connecting devices so that they are not inadvertently dislodged, resulting in a pneumothorax (Fig. 31-11). Verify appropriate placement of the chest drain radiographically (Fig. 31-12) before covering it with a loose bandage. In selected cases (i.e., multiple adhesions, loculated fluid), it may be advantageous to place the chest tube under thoracoscopic guidance. Clip and prepare the lateral thorax for aseptic surgery. Make a small skin incision in the dorsal one third of the lateral thoracic wall at the level of the ninth or tenth intercostal space (Fig. 31-13, A). Tunnel the introducer catheter to the seventh or eighth intercostal space, and insert it at the cranial aspect of the rib into the thoracic cavity (Fig. 31-13, B, C). Advance the introducer into the thorax over the stylet, and thread a J-wire through the catheter. Advance the wire in a cranioventral direction until resistance is encountered (Fig. 31-13, D). Remove the catheter over the guide wire, leaving the guide wire in place. Insert a small-bore catheter into the thoracic cavity over the guide wire. Gently aspirate the drain to assess accurate placement. Secure the drain to the skin through the suture holes on the catheter. If the tube cannot be fully inserted into the thoracic cavity (e.g., in cats or small dogs), secure the external portion of the tube to the skin using a Chinese finger-trap suture (see Fig. 31-10). If using a vascular access port (see p. 998), the VAP-drain combination may be placed via a regular intercostal thoracotomy at the time of surgical exploration of the thoracic cavity or via a small “mini” thoracotomy (see Fig. 31-6). Drainage may be either intermittent or continuous. Generally, intermittent pleural drainage is adequate; however, in some situations continuous suction is preferable (i.e., large volumes of air, pleurodesis). Heimlich valves should be used only in medium to large dogs because small dogs and cats may not develop sufficient expiratory pressure for effective drainage. Also, these valves are prone to malfunction if fluid is aspirated into the apparatus (Salci et al, 2009). “Milking” or “stripping” of chest tubes to prevent obstruction of the tube by clots has been recommended in the veterinary literature; however, these techniques generate high intrapleural pressures and may cause pulmonary damage. Because the tube is a foreign body, it will cause fluid to be produced in most animals (generally 2.2 ml/kg of body weight/day). With pleural effusion, tubes can generally be removed when the volume of fluid is compatible with that which can be managed by intermittent thoracentesis. A correlation between the volume of fluid produced at the time of chest drain removal and time to discharge or the between the time to discharge and the reason for drain placement was not found in a recent study (Margues et al, 2009). The tube can be removed in patients with pneumothorax once negative pressure has been achieved for 12 to 24 hours. Culture the end of the tube after removal if the tube has been in place for several days or if the animal shows signs of infection. Close the skin incision with one or two simple interrupted sutures. If fluid accumulates so quickly that intermittent drainage is not practical, continuous suction may be used. It is also commonly used after open-heart surgery to monitor thoracic bleeding. Two-bottle and three-bottle systems and commercial suction units are available for veterinary use and are economical and simple to use. A continuous 10- to 15-cm negative pressure on the thorax effectively aspirates pneumothorax, increasing the likelihood of spontaneous sealing of large pulmonary defects. Slightly higher pressures may be necessary (up to 20 cm H2O) when viscous fluid is being drained. Connect the chest tube to a commercial continuous suction device (Fig. 32-14). If a commercial continuous suction device is not available, use a “three-bottle” system. Connect the chest tube to a bottle that serves as an underwater seal (filled with 2 to 3 cm of sterile water), which in turn is connected to a suction bottle (also partly filled with water) attached to a suction device (Fig. 31-15). Vary the amount of suction by raising or lowering the level of water in the suction bottle. Healing or damaged pleurae is prone to adhesion formation in some species; however, dogs and cats seem resistant to chemical pleurodesis. They may have greater pleural fibrinolytic capacity than other species (e.g., rabbits or human beings). Fibrosing pleuritis has been reported in dogs and cats secondary to prolonged exudative or blood-stained effusions. In animals with fibrosis, the pleura is thickened by diffuse, fibrous tissue that restricts normal pulmonary expansion (the lungs do not adhere to the body wall in these patients; Fig. 31-16). Exudates are characterized by a high rate of fibrin formation and degradation because chronic inflammation induces changes in the morphologic features of mesothelial cells that cause increased permeability, desquamation of mesothelial cells, and triggering of both pathways of the coagulation cascade. The desquamated mesothelial cells have also been shown to produce type III collagen in cell culture, promoting fibrosis. Additionally, the chronic presence of pleural fluid might lead to impaired fibrin degradation. Trocar chest tubes (DekNatel thoracic trocar catheter, Argyle thoracic trocar catheter) and continuous suction devices (DekNatel Pleur-Evac chest drainage system, Thora-Seal III three-bottle underwater chest drainage system) (see Fig. 31-14) may be purchased from several commercial sources. Tubing clamps and tube adaptors may be purchased from Global Veterinary Products (formerly Cook Veterinary Products) and other sources. Small-bore wire-guided catheters may be purchased from MILA International, Inc., of Erlanger, Kentucky. If dyspnea persists after needle thoracentesis or chest tube placement, oxygen therapy (nasal insufflation or oxygen cage) may be beneficial (see Chapter 4). Thoracic radiographs should be taken to assess fluid or air removal and/or to evaluate the position of the chest tube. Animals with chest tubes should be monitored continually to prevent iatrogenic dislodgment or damage to the tube or connectors, resulting in pneumothorax (discussed later under Complications). Care should be exercised when handling tubes to prevent thoracic contamination. Chest tubes should be aspirated gently so that pulmonary tissue is not suctioned into the tube drainage ports. Although lung penetration and damage are possible with needle thoracentesis, risk is minimal if proper technique is used. The major complication associated with chest tubes is pneumothorax caused by damage to the tube by the patient (i.e., biting or scratching) or loosening of the connections of the tube to the adapters. Risk of these complications can be minimized by placing a clamp close to the tube’s exit site (see Fig. 31-9), by securing the tube to the adapters (see Fig. 31-11), and by proper bandaging of the chest and tube. Constant surveillance of animals with chest tubes is recommended. Other complications associated with chest tube placement are rare but include lung perforation, empyema, laceration of intercostal vessel, and pulmonary injury caused by aspiration of a portion of lung into a tube drainage port. Risk of lung perforation is related to the type of tube placed and underlying pleuropulmonary disease. If needle thoracentesis or ultrasonography suggests that the fluid is severely loculated or that extensive adhesions are present, surgical or thoracoscopic placement of the chest tube may be advisable. Surgical correction of respiratory abnormalities in young animals requires special attention to their anesthetic requirements (see p. 972). Diaphragmatic hernia repair is commonly performed in young animals because they are prone to trauma producing such lesions. Peritoneopericardial diaphragmatic hernias usually are diagnosed at a young age (i.e., less than 1 year), and concurrent cardiac abnormalities may be present, complicating the anesthetic management of these patients (see p. 1008). Geriatric animals may have severe, concurrent underlying pulmonary or cardiac conditions complicating management of pleural cavity disease, and careful monitoring is necessary. Bellegeer, EA, Adams, WM, Dubielzig, RR, et al. Computed tomography characteristics of canine tracheobronchial lymph node metastasis. Vet Radiol Ultrasound. 2010;51:397. Cahalane, AK, et al. Use of vascular access ports in femoral veins of dogs and cats with cancer. J Am Vet Med Assoc. 2007;231(9):1354. DeRycke, LM, Gielen, IM, Simoens, PJ, van Bree, H. Computed tomography and cross-sectional anatomy of the thorax in clinically normal dogs. Am J Vet Res. 2005;66:512. Margues, AIDC, Tattersall, J, Shaw, DJ, Welsh, E. Retrospective analysis of the relationship between time of thoracostomy drain removal and discharge time. J Small Anim Pract. 2009;50:162. McNally, EM, Robertson, SA, Pablo, LS. Comparison of time to desaturation between preoxygenated and nonpreoxygenation dogs following sedation with acepromazine maleate and morphine and induction of anesthesia with propofol. Am J Vet Res. 2009;70:1333. Salci, H, Bayram, AS, Gorgul, OS. Outcomes of Heimlich valve drainage in dogs. Aust Vet J. 2009;87:148. Valtolina, C, Adamantos, S. Evaluation of small-bore wire-guided chest drains for management of pleural space disease. J Small Anim Pract. 2009;50:290. Specific Diseases Animals with recent traumatic diaphragmatic hernias frequently are in shock when presented for treatment; therefore, clinical signs may include pale or cyanotic mucous membranes, tachypnea, tachycardia, and/or oliguria. Cardiac arrhythmias are common and associated with significant morbidity. Other clinical signs depend on which organs have herniated and may be attributed to gastrointestinal, respiratory, or cardiovascular systems. The liver is the most commonly herniated organ, a condition often associated with hydrothorax caused by entrapment and venous occlusion; however, any organ can be herniated, including the kidney (Katic et al, 2007). Definitive diagnosis of pleuroperitoneal diaphragmatic hernia usually is made by radiography or ultrasonography. If significant pleural effusion is present, thoracentesis may be necessary for diagnostic radiographs. Radiographic signs of diaphragmatic hernia may include loss of the diaphragmatic line, loss of the cardiac silhouette, dorsal or lateral displacement of lung fields, presence of gas or a barium-filled stomach or intestines in the thoracic cavity, pleural effusion, and/or failure to observe the stomach or liver in the abdomen (Figs. 31-17 and 31-18). It may be difficult to diagnose diaphragmatic hernias radiographically if only a small portion of the liver is herniated. Ultrasound examination of the diaphragmatic silhouette may help when herniation is not obvious radiographically (i.e., hepatic herniation, pleural effusion). Ultrasonography accurately demonstrates diaphragmatic hernia in the majority of patients. Ultrasonography may be particularly difficult if severe pulmonary contusions are present, which make the lung appear ultrasonographically similar to the liver, if only the omentum is herniated, or if adhesions between the liver and lung are present. Care should be taken not to mistake a normal mirror-image artifact (usually seen as apparent liver parenchyma on the thoracic side of the diaphragmatic line) for herniated liver. If the animal is dyspneic, oxygen should be provided by face mask, nasal insufflation, or oxygen cage (see Chapter 4). Positioning the animal in sternal recumbency with the forelimbs elevated may help ventilation. If moderate or severe pleural effusion is present, thoracentesis (see p. 996) should be performed. Fluid therapy and antibiotics should be given if the animal is in shock. Because of the animal’s already compromised ventilation, drugs with minimal respiratory depressant effects should be used (Table 31-4). Premedication in severely distressed patients may not be advisable. Patients that have minimal dyspnea can be given a benzodiazepine (e.g., midazolam or diazepam). Supplementing oxygen before induction improves myocardial oxygenation, and preoxygenation for 3 to 5 minutes allows for a safer induction. Chamber or mask induction should be avoided in animals with diaphragmatic hernia (see p. 1004). Gentle and quiet handling of the animal may allow surgical clipping prior to induction, minimizing the delay from time of induction to the surgical incision. Anesthetic Considerations for the Diaphragmatic Hernia Patient *Monitor for hyperthermia in cats. Injectable anesthetics allowing rapid intubation are preferred (see also p. 992). Inhalation anesthetics should be used for maintenance of anesthesia. Intermittent positive pressure ventilation should be performed, and high inspiratory pressures should be avoided if possible. However, once the animal is placed in a dorsal position causing a shift of abdominal organs into the chest, higher pressures may be needed to accomplish ventilation. It is important to understand that with severe compression of the lungs, the alveoli are collapsed and the bronchioles and bronchi also may be compressed. In this situation, the airway structures experiencing the higher ventilator pressures are primarily the bronchial tree. Once the chest is opened, allowing room for pulmonary expansion, peak airway pressures should immediately be decreased and the lungs slowly expanded. This slow expansion helps to reduce the chance of reexpansion pulmonary edema (see p. 961). Nitrous oxide is contraindicated in patients with diaphragmatic hernia (see p. 959). Drugs such as methylprednisolone may be beneficial for preventing reexpansion pulmonary edema in animals with chronic diaphragmatic hernia. Adequate pain management is especially important in respiratory compromised patients. Postoperatively, these patients need to be able to take slow deep breaths; however, a painful patient will take rapid and shallow breaths. Epidural, intercostal nerve blocks, incisional local block, and intravenous opioids may be used in combination to minimize pain (see Box 31-2 on p. 992 and Table 31-3 on p. 994). Make a ventral midline abdominal incision; if greater exposure is needed, extend the incision cranially through the sternum. Replace the abdominal organs in the abdominal cavity (if necessary, enlarge the diaphragmatic defect). If adhesions are present, dissect the tissues gently from the thoracic structures to prevent pneumothorax or bleeding. With chronic hernias, debride the edge of the defect before closure. Close the diaphragmatic defect in a simple continuous suture pattern. If the diaphragm is avulsed from the ribs, incorporate a rib in the continuous suture for added strength (Fig. 31-19). Remove air from the pleural cavity after closing the defect. If continued pneumothorax or effusion is likely, place a chest tube (see p. 997). Explore the entire abdominal cavity for associated injury (i.e., compromise of the vasculature to the intestine or splenic, renal, or bladder trauma), and repair any defects. FIG 31-19 To repair a diaphragm avulsed from the thoracic wall, incorporate a rib in the suture line. Patients should be monitored postoperatively for hypoventilation, and oxygen should be provided if necessary. Reexpansion pulmonary edema (RPE) is a possible complication associated with rapid lung reexpansion after repair of a diaphragmatic hernia (see p. 961). Postoperative analgesics should be provided (see Chapter 12; for dosages of opioids, see Table 12-3 on p. 141). The prognosis is excellent if the animal survives the early postoperative period (i.e., 12 to 24 hours), and recurrence is uncommon with proper technique. Reported mortality rates for animals with traumatic diaphragmatic hernia vary from 12% to 48%. Perioperative survival in one study of 92 dogs and cats with traumatic diaphragmatic hernias was 89.1% (Gibson et al, 2005). Ten to twenty percent of chronic diaphragmatic hernias may die. Older cats or those that have low to mildly increased respiratory rates and concurrent injuries may be more likely to die after hernia repair. Gibson, TWG, Brisson, BA, Sears, W. Perioperative survival rates after surgery for diaphragmatic hernia in dogs and cats. J Am Vet Med Assoc. 2005;227:105. Katic, N, Bartolomaeus, E, Boohler, A, Dupre, G. Traumatic diaphragmatic rupture in a cat with partial kidney displacement into the thorax. J Small Anim Pract. 2007;48:705. Peritoneopericardial Diaphragmatic Hernia Peritoneopericardial diaphragmatic hernias are less commonly recognized by small animal clinicians than traumatic diaphragmatic hernias. Although PPDHs often are associated with respiratory embarrassment, asymptomatic PPDH is common. PPDH may occur as a result of trauma in human beings (in whom the diaphragm forms one wall of the pericardial sac); however, these hernias are always congenital in dogs and cats because no direct communication exists between the pericardial and peritoneal cavities after birth. The most widely accepted theory regarding the embryogenesis of this defect is that the hernia occurs because of faulty development or prenatal injury of the septum transversum. This could be a result of a teratogen, genetic defect, or prenatal injury. True diaphragmatic hernias have recently been reported in two dogs (Choi et al, 2009). Cardiac abnormalities and sternal deformities often occur concomitantly with PPDH. The combination of congenital cranial abdominal wall, caudal sternal, diaphragmatic, and pericardial defects has been reported in dogs, often associated with ventricular septal defects or other intracardiac defects (Fig. 31-20). It is not known if this condition is heritable; however, several breed predispositions have been recognized (discussed later). Polycystic kidneys have been reported in association with PPDH in cats.
Surgery of the Lower Respiratory System
Pleural Cavity and Diaphragm
Preoperative Concerns
![]() TABLE 31-1
TABLE 31-1
HR/MINUTE
RR/MINUTE
Dog
70-140
20-40
Cat
145-200
20-40
![]() TABLE 31-2
TABLE 31-2
VALUE
RANGE
pH
7.4
(7.35-7.45)
Pao2
95 mm Hg
(80-110)
Pvo2
40 mm Hg
(35-45)
Paco2
40 mm Hg
(35-45)
Pvco2
45 mm Hg
(40-48)
Hco3−
24 mEq/L
(22-27)
Anesthetic Considerations
![]() TABLE 31-3
TABLE 31-3


Antibiotics
Surgical Anatomy
Surgical Techniques
Needle Thoracentesis
Chest Tube Placement
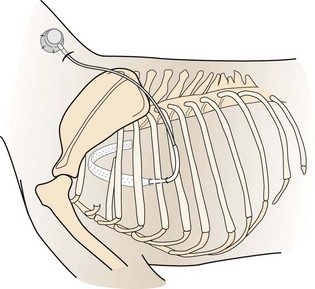
Trocar and large bore chest tubes
Small-bore wire-guided chest tubes
Chest Tube Removal
Continuous Thoracic Suction
Healing of the Pleura
Suture Materials and Special Instruments
Postoperative Care and Assessment
Complications
Special Age Considerations
References
Traumatic Diaphragmatic Hernia
Diagnosis
Physical Examination Findings
Diagnostic Imaging
Medical Management
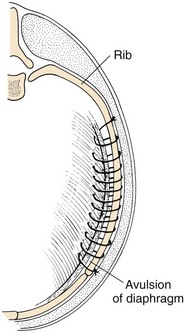
Surgical Treatment
Anesthesia
![]() TABLE 31-4
TABLE 31-4


Surgical Technique
Postoperative Care and Assessment
Prognosis
References
General Considerations and Clinically Relevant Pathophysiology
Surgery of the Lower Respiratory System: Pleural Cavity and Diaphragm