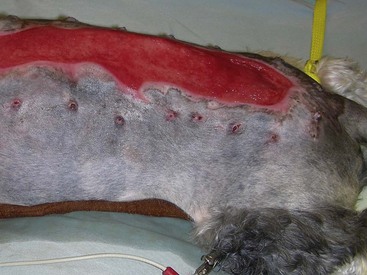
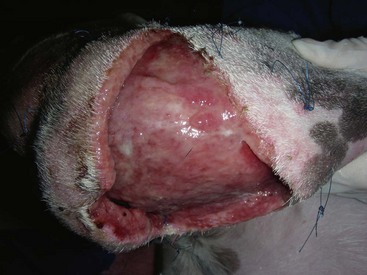
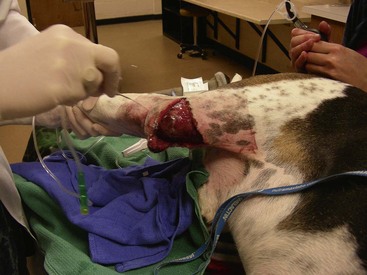
Chapter 16 General Principles and Techniques Inflammatory Phase: Inflammation is a protective tissue response initiated by damage. This phase is characterized by increased vascular permeability, chemotaxis of circulatory cells, release of cytokines and growth factors, and cell activation (macrophages, neutrophils, lymphocytes, and fibroblasts). Hemorrhage cleans and fills wounds immediately after injury. Blood vessels constrict for 5 to 10 minutes to limit hemorrhage, but they then dilate and leak fibrinogen and clotting elements into wounds. Vasoconstriction is mediated by catecholamines, serotonin, bradykinin, and histamine. The extrinsic coagulation mechanism is activated by thromboplastin released from injured cells. Platelet aggregation and blood coagulation form a clot that ensures hemostasis and provides a scaffold for cell migration. Platelets also release potent chemoattractants and growth factors (epidermal, platelet-derived, transforming growth factors: α and β) that are necessary in later stages of wound healing (Box 16-1). Fibrin and plasma transudates fill wounds and plug lymphatics, localizing inflammation and “gluing” wound edges together. Fibronectin dimers within the clot become covalently cross-linked to fibrin and to themselves in the presence of activated factor XIII, forming a provisional extracellular matrix. This blood clot formation stabilizes the wound’s edges and provides limited wound strength. It also provides an immediate barrier to infection and fluid loss, and a substrate for early organization of the wound. Scabs form when the blood clot dries; they protect wounds, prevent further hemorrhage, and allow healing to progress beneath their surface. Inflammatory phase cells such as platelets, mast cells, and macrophages secrete growth factors or cytokines, which initiate and maintain the proliferative phase of healing. Inflammatory mediators (i.e., histamine, serotonin, proteolytic enzymes, kinins, prostaglandins, complement, lysosomal enzymes, thromboxane, and growth factors) cause inflammation that begins immediately after injury and lasts approximately 5 days. White blood cells leaking from blood vessels into wounds initiate the débridement phase. Débridement Phase: An exudate composed of white blood cells, dead tissue, and wound fluid forms on wounds during the débridement phase. Chemoattractants encourage neutrophils and monocytes to appear in wounds (approximately 6 hours and 12 hours after injury, respectively) and initiate débridement. Neutrophils increase in number for 2 to 3 days. They prevent infection and phagocytize organisms and debris. Degenerating neutrophils release enzymes and toxic oxygen products that facilitate breakdown of bacteria, extracellular debris, and necrotic material, and they stimulate monocytes. Monocytes are essential for wound healing; neutrophils are not. Monocytes are major secretory cells synthesizing growth factors that participate in tissue formation and remodeling. Monocytes become macrophages in wounds at 24 to 48 hours. Macrophages secrete collagenases removing necrotic tissue, bacteria, and foreign material. They may coalesce and form multinucleated giant cells with phagocytic functions. Macrophages also secrete chemotactic and growth factors. Growth factors (i.e., platelet-derived growth factor, transforming growth factor-α, transforming growth factor-β, fibroblast growth factor, and interleukin-1) can initiate, maintain, and coordinate formation of granulation tissue. Chemotactic factors (i.e., complement, collagen fragments, bacterial endotoxins, and inflammatory cell products) direct macrophages to injured tissue. Macrophages also recruit mesenchymal cells, stimulate angiogenesis, and modulate matrix production in wounds. Platelets release growth factors important for fibroblastic activity. Lymphocytes appear later in the débridement phase than neutrophils and macrophages. They secrete soluble factors that may stimulate or inhibit migration and protein synthesis by other cells. However, they usually improve the rate and quality of tissue repair. Although healing is severely impaired when macrophage function is suppressed, neutropenia and lymphopenia do not inhibit healing or the development of wound tensile strength in sterile wounds. Repair Phase: The repair phase usually begins 3 to 5 days after injury. Macrophages stimulate deoxyribonucleic acid (DNA) and fibroblast proliferation. Cytokines, in concert with extracellular matrix molecules, stimulate fibroblasts in the surrounding tissue to proliferate, express appropriate integrin receptors, and migrate into wounds. Fibroblasts are stimulated by transforming growth factor-β to produce fibronectin, which facilitates cell binding and fibroblast movement. Platelet-derived growth factor and basic fibroblast growth factor are also involved. A tissue oxygen content of approximately 20 mm Hg and slight acidity also stimulate fibroblast proliferation and collagen synthesis. Fibroblasts originate from undifferentiated mesenchymal cells in surrounding connective tissue and migrate to wounds along fibrin strands in the fibrin clot. Fibroblasts migrate into wounds just ahead of new capillary buds as the inflammatory phase subsides (2 to 3 days). They invade wounds to synthesize and deposit collagen, elastin, and proteoglycans that mature into fibrous tissue. Orientation initially is haphazard, but after 5 days tension on wounds causes fibroblasts, fibers, and capillaries to orient parallel to the incision or wound margin. Wound fibrin disappears as collagen is deposited. Collagen synthesis is associated with an early increase in wound tensile strength. As the wound matures, there is a notable increase in the ratio of type I (mature) to type III (immature) collagen. The amount of collagen reaches a maximum within 2 to 3 weeks after injury. As the collagen content of a wound increases, the number of fibroblasts and the rate of collagen synthesis decrease, marking the end of the repair stage. The fibroblastic interval of healing lasts 2 to 4 weeks, depending on the nature of the wound. Fibroblast migration and proliferation, collagen production, and capillary ingrowth are delayed if macrophages are absent. Granulation tissue is formed at each wound edge at a rate of 0.4 to 1 mm/day. Unhealthy granulation tissue is white and has a high fibrous tissue content with few capillaries (Figs. 16-1 and 16-2). Granulation tissue fills defects and protects wounds. It provides a barrier to infection, a surface for epithelial migration, and a source of special fibroblasts (i.e., myofibroblasts), which are important in wound contraction. Myofibroblasts are believed to contain proteins (actin and myosin) that contribute to wound contraction. Myofibroblasts are not found in normal tissue, incised and coapted wounds, or tissue surrounding a contracting wound. FIG 16-1 An open wound with unhealthy granulation over the right stifle in a 4-year old Walker Hound. Epithelium is an important barrier to external infection and internal fluid loss. Epithelial repair involves mobilization, migration, proliferation, and differentiation of epithelial cells. Epithelialization begins almost immediately (24 to 48 hours) in sutured wounds with good edge to edge apposition because there is no defect for granulation tissue to fill (see Fig. 16-2). Epithelialization begins in open wounds when an adequate granulation bed has formed (usually 4 to 5 days). In partial-thickness skin wounds, epidermal migration over the wound surface begins almost immediately from both the wound margins and epidermal appendages, such as hair follicles and sweat glands. Epidermal cells at the margin of the wound undergo phenotypic alteration that includes retraction on intracellular monofilaments, formation of peripheral cytoplasmic actin filaments, and temporary dissolution of the desmosomes and hemidesmosomes, which release keratinocytes to migrate beneath the eschar at the junction between any remaining necrotic tissue and extracellular matrix of the viable connective tissue. The epidermal cell path of migration is determined by integrins expressed on the membranes of migrating epidermal cells. Chalone, water-soluble glycoproteins found in the epidermis, inhibits epithelial mitosis in normal tissue but is diminished in wounds, which allows epithelial cells along wound margins to divide and migrate across the granulation tissue. Other growth factors secreted by platelets, macrophages, and fibroblasts may also be involved. Increased basal cell mitotic activity occurs as early as 24 to 48 hours after wounding. Epithelial migration is random but guided by collagen fibers. Migrating epithelial cells enlarge, flatten, and mobilize, losing their attachments to the basement membrane and other epithelial cells. Basal cells at wound edges develop microvilli and extend broad, thin pseudopodia over the exposed surface of collagen bundles. They develop intracytoplasmic microfilaments and selectively fix antiactin and antimyosin antibodies. Epithelial cells in the layers behind these altered cells migrate over them until they contact the wound surface. Cells continue to slide forward until the wound surface is covered. The migrating cells move under scabs and produce collagenase, which dissolves the base of the scab so it can be shed. Contact on all sides with other epithelial cells inhibits further cell migration (contact inhibition). Initially, new epithelium is only one cell layer thick and fragile, but it gradually thickens as additional cell layers form. After a basement membrane has been established, epithelial cells become plump, develop mitoses, and proliferate, restoring the normal, stratified, squamous epithelium architecture. Some hair follicles and sweat glands may regenerate, depending on the depth of skin damage. Epithelial migration also occurs along suture tracts, which may lead to a foreign body reaction, sterile abscess, or scarring or all of these. Epithelialization of suture tracts can be minimized by early removal of sutures. New epithelium usually is visible 4 to 5 days after injury. Epithelialization occurs faster in a moist environment than in a dry one. It will not occur over nonviable tissue. Epithelial migration is energy-dependent and related to oxygen tension. Anoxia prevents epithelial migration and mitosis, whereas hyperbaric oxygen therapy may enhance migration. Wet-dry bandages (see p. 216) débride newly formed epithelium, delaying reepithelialization. Maturation Phase: Wound strength increases to its maximum level because of changes in the scar during the maturation phase of wound healing. Wound maturation begins once collagen has been adequately deposited in wounds (17 to 20 days after injury) and may continue for years. The cellularity of granulation tissue is reduced as cells die. There is also a reduction in collagen content of the extracellular matrix. Collagen fibers remodel with alteration of their orientation and increased cross-linking, which improves wound strength. Fibers orient along lines of stress. Functionally oriented fibers become thicker. Type III collagen gradually decreases, and type I collagen increases. Nonfunctionally oriented collagen fibers are degraded by proteolytic enzymes (matrix metalloproteinases) secreted by macrophages, epithelial cells, endothelial cells, and fibroblasts within the extracellular matrix. The most rapid gain in wound strength occurs between 7 and 14 days after injury as collagen rapidly accumulates in the wound. Wounds gain only about 20% of their final strength in the first 3 weeks after injury. Slower increase in wound strength then occurs, but normal tissue strength is never regained in wounds; only 80% of original strength may be regained. As the number of capillaries in fibrous tissue declines, the scar becomes paler. Scars also become less cellular, flatten, and soften during maturation. Collagen synthesis and lysis occur at the same rate in maturing scars. Cutaneous wounds are slower to heal in cats than dogs. Sutured wounds in cats are only half as strong as similar wounds in dogs after 7 days of healing. Cat wounds that are allowed to heal by secondary intention heal slower, produce less granulation tissue (that is more peripherally located), and heal more by contraction of wound edges than dogs (Bohling et al, 2004). Radiation therapy (see p. 262) and some drugs delay wound healing. Corticosteroids depress all phases of wound healing and increase chances of infection. Vitamin A and anabolic steroids may reverse the effects of corticosteroids on wound healing. Anti-inflammatory drugs suppress inflammation but have little effect on wound strength. Aspirin may delay blood clotting. Some chemotherapeutic drugs (e.g., cyclophosphamide, methotrexate, and doxorubicin) inhibit wound healing. Radiation therapy can profoundly inhibit wound healing, depending on dose and time of exposure relative to the time of injury. It reduces the quantity of blood vessels, affects collagen maturation, and causes increased dermal fibrosis. Therefore, chemotherapeutic drugs and radiation therapy should be avoided for 2 weeks after surgery. Vitamin A, vitamin E, and aloe vera may promote healing in irradiated wounds. Exposure to pico-tesla electromagnetic field treatment improves strength of sutured wounds and speeds contraction of open wounds in rats. Hyperbaric oxygen therapy increases dissolved oxygen in plasma, which stimulates growth of new capillaries; therefore, it may be useful for treatment of ischemic wounds. Ultrasonography and phototherapy (low-powered laser) shorten the inflammatory phase of healing and enhance release of factors that stimulate the proliferative stage of repair. Use of controlled subatmospheric pressure dressings helps remove interstitial fluid, which allows tissue decompression, helps remove tissue debris, and promotes wound healing (see p. 205). Wounds should be covered with a clean, dry bandage immediately after injury or when the animal is brought for treatment to prevent further contamination and hemorrhage (Box 16-2). Life-threatening injuries should be treated and the animal’s condition stabilized before further wound management is undertaken. When appropriate during stabilization, bandages should be removed and the wound assessed and classified as either contaminated or infected and as an abrasion, laceration, avulsion, puncture, crush, or burn wound. The “golden period” is the first 6 to 8 hours between wound contamination at injury and bacterial multiplication to greater than 105 CFU per gram of tissue. A wound is classified as infected rather than contaminated when bacterial numbers exceed 105 CFU per gram of tissue. Infected wounds often are dirty and covered with a thick, viscous exudate. Abrasions are superficial and involve destruction of varying depths of skin by friction from blunt trauma or shearing forces. Abrasions are sensitive to pressure or touch and bleed minimally. A laceration is created by tearing, which damages skin and underlying tissue. Lacerations may be superficial or deep and have irregular edges. Avulsion wounds are characterized by the tearing of tissues from their attachments and the creation of skin flaps. Avulsion injuries on limbs with extensive skin loss are called degloving injuries. A penetrating or puncture wound is created by a missile or sharp object, such as a knife, pellet, or tooth that damages tissue. Wound depth and width vary depending on the velocity and mass of the object creating the wound. The extent of tissue damage is directly proportional to missile velocity. Pieces of hair, skin, and debris can be embedded in wounds. Crush injuries can be a combination of other types of wounds with extensive damage and contusions to skin and deeper tissue. Burns may be partial- or full-thickness skin injuries caused by heat or chemicals (see p. 257). Wounds less than 6 to 8 hours old with minimal trauma and minimal contamination are treated by lavage, débridement, and primary closure. Generally, the sooner treatment begins, the better the prognosis. Penetrating wounds should not be primarily apposed without surgical exploration (see p. 265). Severely traumatized and contaminated wounds, wounds older than 6 to 8 hours, or infected wounds should be treated as open wounds to allow débridement and reduction of bacterial numbers. Most wounds are surgically apposed after infection has been controlled; however, some wounds heal by contraction and epithelialization (healing by secondary intention). Initial wound management begins with removal of gross contaminants and copious lavage using a warm, balanced electrolyte solution, sterile saline, or tap water (500 to 1000 ml) (Table 16-1). Sterile isotonic saline or a balanced electrolyte solution (lactated Ringer’s solution) is the preferred lavage solution. Tap water is effective and less detrimental than distilled or sterile water, although it causes some hypotonic tissue damage (cellular and mitochondrial swelling). Wound lavage reduces bacterial numbers mechanically by loosening and flushing away bacteria and associated necrotic debris. Lavage may be facilitated by the use of noncytotoxic wound cleansers (e.g., Allclenz Wound Cleanser, Healthpoint Biotherapeutics, Fort Worth, Tex.). Generally, these cleansers are applied to loosen debris and soften necrotic tissue during bandage changes; they act as a surfactant, disrupting the ionic bonding of particles and organisms to the wound and allowing them to be easily rinsed off with saline or balanced electrolyte solutions. Lavage following application of these cleansers, however, is not necessary. Antibiotics or antiseptics (e.g., chlorhexidine or povidone-iodine; see p. 201) in the lavage solution reduce bacterial numbers; however, these agents may damage tissue. Antiseptics have little effect on bacteria in established infections. Lavaging is preferred to scrubbing the wound with sponges. Sponges inflict tissue damage that impairs the wound’s ability to resist infection and allows residual bacteria to elicit an inflammatory response. Bacteria are effectively removed from the wound surface by high-pressure lavage. Traditionally, a 35- or 60-ml syringe and an 18-gauge needle have been thought to generate approximately 7 to 8 psi of pressure; however, it was recently shown that it generates pressures substantially higher than this (18.4 ± 9.8 psi) (Gall and Monnet, 2010). The most consistent delivery method to generate 7 to 8 psi is a 1-liter bag of fluid within a cuff pressurized to 300 mmHg (Fig. 16-3). Higher pressure (70 psi), generated by pulsatile lavage instruments (i.e., Water Pik [Teledyne], Surgilav, or Pulsavac débridement system) is more effective in reducing bacterial numbers and removing foreign debris and necrotic tissue, but it may drive bacteria and debris into loose tissue planes, damage underlying tissue, and reduce resistance to infection. Bulb syringes or fluid bottles with holes made in the cap do not generate enough pressure to remove bacteria and debris adequately. FIG 16-3 Wound lavage of the dog from Fig. 16-1 using 1 L of 0.9% saline in pressure bag, maintained at 300 mmHg with extension tubing and an 18-gauge needle. Healing is delayed if necrotic tissue is left in the wound. Devitalized tissue is removed from the wound by débridement. Débridement involves removal of dead or damaged tissue, foreign bodies, and microorganisms that compromise local defense mechanisms and delay healing. The goal of débridement is to obtain fresh clean wound margins and wound bed for primary or delayed closure. Devitalized tissue is removed by surgical excision, autolytic mechanisms, enzymes, wet-dry bandages (see p. 216), or biosurgical methods. The extent of devitalized tissue usually is obvious within 48 hours of injury. Surgical Débridement: Devitalized tissue should be surgically excised in layers beginning at the surface and progressing to the depths of the wound. This can be done by sharp dissection, electrosurgery, or laser. Bones, tendons, nerves, and vessels must be preserved, but bone sequestra should be removed because they may prevent complete granulation of the wound (especially with metacarpal and metatarsal degloving injuries) and predispose the wound to infection. Muscle should be débrided until it bleeds and contracts with appropriate stimuli. Extensive debridement of subcutaneous tissue should be avoided as it may delay wound healing, particularly in cats, and may place wounds at a higher risk for infection (Bohling et al, 2006). Contaminated fat should be liberally excised because it is easily devascularized and harbors bacteria, but cutaneous vessels must be spared to maintain the viability of overlying skin. Autolytic Débridement: Autolytic débridement is accomplished through creation of a moist wound environment to allow endogenous enzymes to dissolve nonviable tissue. It is often preferred over surgical or bandage débridement in wounds with questionable tissue viability, as it is highly selective for devitalized tissue and markedly less painful than other methods of debridement; however, it is a much slower process. Autolytic débridement is accomplished with hydrophilic, occlusive, or semiocclusive bandages (see pp. 193 and 211), which allow wound fluid to remain in contact with nonviable tissue. Bandage (Mechanical) Débridement: Dressings that are allowed to dry on the wound, such as wet-to-dry bandages or dry-to-dry bandages, adhere to the wound surface and pull the debris and strip the superficial layers off the wound bed when removed. In addition to mechanical debridement, wet-to-dry wound dressings provide adequate wound protection and coverage, maintain a moist wound environment, and absorb moderate amounts of wound exudates. These dressings are most effective in early stages of wound healing or in the management of wound infection. However, débridement is painful and nonselective, as healthy or healing tissue may be traumatized. Enzymatic Débridement: Enzymatic débriding agents are used as an adjunct to wound lavage and surgical débridement. They are beneficial in patients that are poor anesthetic risks or when surgical débridement may damage healthy tissue necessary for reconstruction. Enzymatic agents break down necrotic tissue and liquefy coagulum and bacterial biofilm, allowing better antibiotic contact with wounds and enhanced exposure for development of cellular and humoral immunity; they do not damage living tissue if used properly. Available enzymes do not digest burned skin, necrotic bone, and connective tissue. Enzymes must remain in contact with the wound for an adequate time to produce the desired effect. Local tissue irritation may occur with enzyme use. Biosurgical Débridement: Maggot therapy using greenbottle fly larvae (Lucilia sericata) débrides wounds as the maggots secrete proteolytic digestive enzymes into the wound. Sterile medicinal maggots (Monarch Laboratories, LLP, Irvine, Calif.) are bred specifically for biosurgery. A single maggot may consume up to 75 mg of necrotic tissue each day. They require an optimal temperature, an oxygen supply, and a moist wound. Maggot therapy is best suited to necrotic, infected, or chronic nonhealing wounds. The maggots remove necrotic tissue, disinfect the wound, and promote granulation tissue formation. Medicinal maggots are applied to the wound at a density of five to eight per square centimeter. A hole is cut into a self-adhesive hydrocolloid dressing that matches the wound dimensions. This dressing is applied to the wound to prevent the maggots from crawling onto intact skin and to absorb wound secretions. The dressing is covered to trap the maggots in the wound, changing absorbent layers as necessary. Maggots are usually applied for two 48-hour cycles each week. Systemic antibiotics should be given if there is a high risk of bacteremia or disseminated infection. A broad-spectrum antibiotic should be administered while awaiting culture results. Antibiotic blood levels should be present at the time of surgery when antibiotics are used prophylactically for clean or clean contaminated procedures. Prophylactic antibiotics optimally are given intravenously when anesthesia is induced (see Chapter 9). Contamination occurring during surgery usually is limited to the patient’s skin flora; therefore, drugs effective against Gram-positive skin flora, especially Staphylococci spp. (see p. 92), should be selected (e.g., 22 mg/kg cefazolin given intravenously). Triple Antibiotic Ointment: Triple antibiotic ointment (bacitracin, neomycin, polymyxin) is effective against a broad spectrum of pathogenic bacteria commonly infecting superficial skin wounds. However, its efficacy against pseudomonads is poor. Zinc bacitracin is responsible for enhancing reepithelialization of wounds but can retard wound contraction. Because these drugs are poorly absorbed, systemic toxicosis (nephrotoxicity, ototoxicity, neurotoxicity) is rare. The ointment is more effective for preventing infections than for treating them. Silver Sulfadiazine: Silver sulfadiazine in a 1% water-miscible cream (Thermazene, Covidien, Mansfield, Mass.; Silvadene, King Pharmaceuticals, Bristol, Tenn.) is effective against most Gram-positive and Gram-negative bacteria and most fungi. It also serves as an antimicrobial barrier, can penetrate necrotic tissue, and enhances wound epithelialization. It is the drug of choice to treat burn wounds. In vitro toxicity to human keratinocytes and fibroblasts and inhibition of polymorphonuclear cells and lymphocytes has been shown. These wound-retardant effects of silver sulfadiazine are reversed when it is combined with aloe vera. Silver-impregnated dressings are also available (see p. 215). Ointments remain effective for up to 3 days, whereas dressings may be left in place for 7 days. Only a small amount of silver is released slowly from its molecular lattice over a sustained period, reducing cytotoxic effects of ionic silver and making it nonstaining, nonirritating, and nonsensitizing while maintaining its antimicrobial effects. These products are also hydrophilic, helping to maintain a moist wound environment and absorbing exudates. Nitrofurazone: Nitrofurazone (Furacin) has broad-spectrum antibacterial and hydrophilic properties. It has little effect against Pseudomonas spp. Its polyethylene base gives it hydrophilic properties, enabling it to draw body fluid from wound tissue, which helps dilute tenacious exudates so they can be absorbed into bandages. Nitrofurazone delays wound epithelialization. It loses some of its antibacterial effects in the presence of organic matter. Gentamicin Sulfate: Gentamicin sulfate is available as a 1% ointment or powder (Garamycin), but solutions are preferred. Products with an oil-in-water cream base slow wound contraction and epithelialization. It is especially effective in controlling Gram-negative bacterial growth (Pseudomonas spp., Escherichia coli, Proteus spp). It is often used before and after grafting and for wounds that have not responded to triple antibiotic ointment. However, gentamicin in an isotonic solution does not inhibit contraction and it promotes epithelialization. Cefazolin: Cefazolin is an effective antimicrobial against Gram-positive and some Gram-negative organisms. Topical cefazolin (the combined systemic and topical dose should not exceed 22 mg/kg) provides high levels of antibiotic in wound fluid. The drug’s minimum inhibitory concentration is prolonged in wounds when it is applied topically compared with systemic administration. Topically administered cefazolin is 95% bioavailable and rapidly absorbed; therefore systemic levels equal wound fluid levels within 1 hour. Mafenide: Mafenide (hydrochloride or acetate) is a topical sulfa compound available as an aqueous spray. It has a broad spectrum against many Gram-positive and Gram-negative bacteria, including Pseudomonas spp., as well as anaerobic strains such as Clostridium spp., and is particularly useful on severely contaminated wounds. Aloe Vera: Aloe vera gel is extracted from the aloe vera leaf and contains 75 potentially active constituents. Aloe vera has been used on burns because of its antibacterial activity against Pseudomonas aeruginosa. It also inhibits fungal growth. The antiprostaglandin and antithromboxane properties of aloe vera medications are beneficial in maintaining vascular patency and thus helping avert dermal ischemia. Aloe vera medications may also stimulate fibroblastic replication. Aloe vera has the ability to penetrate and anesthetize tissue. Acemannan, a component of aloe vera extract gel, promotes wound healing (see later discussion). It is also found in other preparations. Allantoin, another component of aloe vera extract gel, stimulates tissue repair in suppurating wounds and resistant ulcers by promoting epithelial growth. Use on full-thickness wounds is discouraged because of its anti-inflammatory effects. Aloe vera counteracts the inhibitory effects of silver sulfadiazine when the two are combined. Acemannan: Acemannan (Carravet, Veterinary Products Laboratories, Phoenix, Ariz.; Carrasorb, Carrington Laboratories, Irving, Tex.) is available as a topical wound hydrogel or a freeze-dried gel form. It is indicated for managing superficial and deep partial-thickness burns, lacerations, dermal ulcers, abrasions, and nonhealing wounds. Acemannan is a β-(1,4) acetylated mannan derived from the aloe vera plant that enhances early stages of healing. Acemannan stimulates macrophages to secrete interleukin-1 and tumor necrosis factor alpha, which enhance fibroblast proliferation, neovascularization, epidermal growth and motility, and collagen deposition to form granulation tissue. Acemannan may also bind growth factors, prolonging their stimulating effect on formation of granulation tissue. The freeze-dried form enhances healing over exposed bone and has hydrophilic properties, which help cleanse the wound and reduce wound edema. The most effective time to begin topical application is in the early inflammatory stage of healing with daily application under a bandage, continuing into the repair stage of healing. The greatest effects are seen in the first 7 days of application. Excess granulation tissue can occur, especially with the freeze-dried form, which inhibits wound contraction. Tripeptide-Copper Complex: Glycyl-L-histidyl-lysine tripeptide-copper complex (Iamin-Vet Skin Care Gel, Procyte, Covington, Ga.) stimulates wound healing and is a chemoattractant for mast cells, monocytes, and macrophages, which stimulate débridement, angiogenesis, collagen synthesis, and epithelialization. Enzymes involved in collagen cross-linking need copper. The best time to begin tripeptide-copper complex application is the late inflammatory and early repair phases, with treatment continuing into the later repair phase. It has been effective in accelerating wound healing in chronic, ischemic open wounds. Its greatest effect is in the first 7 days of its use. Exuberant granulation tissue may be a problem with this agent. D-Glucose Polysaccharide (Maltodextrin NF): Maltodextrin (Intracell, Macleod Pharmaceutical, Fort Collins, Colo.) is available in a hydrophilic powder or gel form containing 1% ascorbic acid for use on contaminated and infected wounds as a wound healing stimulant. It is reported to stimulate healing by supplying glucose for cell metabolism via hydrolysis of its polysaccharide component. Its hydrophilic property draws fluid through the tissue, keeping it moist. Maltodextrin is chemotactic and pulls neutrophils, lymphocytes, and macrophages into the wound. Maltodextrin also has antibacterial and bacteriostatic properties. It reduces odor, exudates, swelling, and infection, and it may enhance early granulation tissue formation and epithelialization. After débridement and lavage, a 5- to 10-mm layer of maltodextrin is applied to the wound and covered with a bandage from the early inflammatory stage into the repair stage of healing. Daily bandage changes, lavage, and reapplication are recommended. Honey: Honey is an old agent that has seen renewed interest. Proposed benefits include enhancing wound débridement, reducing edema and inflammation, promoting granulation tissue formation and epithelialization, and improving wound nutrition. It has a wide antibacterial effect through its enzymatic production of hydrogen peroxide from glucose, hypertonicity, low pH, inhibin content, and other unidentified components. Honey increases collagen content, accelerates collagen maturation resulting from cross-linking, and maintains optimal pH conditions for fibroblast activity. Honey contains a wide range of amino acids, vitamins, and trace elements in addition to readily assimilable sugars that stimulate tissue growth. Honey should be used early in the course of wound healing and discontinued once a healthy granulation bed is present. Honey can also be used for treating partial-thickness burns and may have greater wound healing properties than silver-based preparations (Malik et al, 2010). Sugar: Sugar has similar hypertonic effects to honey and also attracts macrophages, accelerates sloughing of devitalized tissue, provides a cellular energy source, and promotes formation of a healthy granulation bed. Granulated sugar is applied in a 1-cm-thick layer, and absorbent bandages are placed to absorb the excess wound fluid. The bandage is changed one to three times a day depending on the amount of strikethrough. Alternatively, a sugar paste made of castor sugar, icing sugar, glycerin, and hydrogen peroxide can be used instead of granulated sugar. Growth Factors: Application of growth factors to stimulate more rapid healing has been investigated. Application of growth factors assumes the wound is deficient in specific growth factors. Knowing which factor is deficient in what amount at what time is nearly impossible in the complex healing process. Evidence indicates that applying single growth factors to wounds is not as effective as the combination of growth factors that the body produces. Allowing these factors to remain on the wound in the wound fluid under an occlusive or semiocclusive bandage is preferred to adding exogenous growth factors. A few growth factors are available commercially including recombinant human-derived platelet-derived growth factor (Regranex, Johnson & Johnson, Arlington, Tex.), but efficacy studies in dogs and cats have yet to be performed. Hydrolyzed Bovine Collagen: Hydrolyzed bovine collagen (HyCure powder and Collasate gel or spray, Hymed, Bethlehem, Pennsylvania) has hydrophilic properties that help create a moist environment for autolytic debridement in early phases of wound healing and an optimal environment for epithelialization in latter stages. There is little histologic evidence of an inflammatory reaction in dogs. The collagen matrix provided serves as a lattice for ingrowth of fibroblasts, thus facilitating the repair phase of wound healing. It is probably most effective when used in the late inflammatory and early repair phases of healing to accelerate epithelialization. Chitosan: Chitosan is a polysaccharide derived from the chitin exoskeleton of shellfish. The active ingredient, glucosamine, is reported to enhance the function of inflammatory cells, growth factors, and fibroblasts to promote formation of granulation tissue and accelerate wound healing. Information regarding the use of chitosan in veterinary medicine is currently scarce. Other Topical Wound Agents: Anti-inflammatory agents are often used to prevent progressive inflammatory damage; however, topical steroids may inhibit epithelialization, wound contraction, and angiogenesis. Production of exuberant granulation tissue may be reduced by one or two applications of corticosteroids. Topical anesthetics may be used to reduce the animal’s pain. Lidocaine or bupivacaine applied topically reduces traumatic and postoperative pain and may decrease the need for systemic analgesics. Hydrophilic agents cause diffusion of fluids through wound tissues to the surface or into the bandage. This dilutes the tenacious coagulum and debris on the wound surface and allows easier absorption. An organic acid combination of malic, benzoic, and salicylic acids (Derma Clens, Pfizer, New York, N.Y.) enhances fluid absorption by devitalized tissue to promote its separation from wounds. Acids do not damage underlying healthy tissue, and the 2.8 pH discourages microbial growth. Hexamethyldisiloxane acrylate copolymer (Cavilon, No Sting Barrier Film; 3M, St. Paul, Minn.) produces a uniform, transparent, colorless, fast-drying, noncytotoxic film that serves as a skin protectant type of dressing. Applied in a thin layer to clean, dry skin every third day, it allows inflammation to resolve quickly and prevents tape from causing epidermal striping and skin irritation. Hydroxyethylated amylopectin (Facilitator; Ridge Pharmaceuticals, Idexx Laboratories, Greensboro, N.C.) is a similar product used in place of a bandage on superficial lesions. This water-soluble product is placed in a thin layer (one drop spread over 2 square inches) over the wound and allowed to dry. It reduces wound drying and itching and accelerates healing. Neither of these products should be used with other topicals as they may inhibit adherence of the film to the skin. Wound cleansing solutions should have ideal antiseptic properties with minimal cytotoxicity. They are used primarily in the initial phases of wound management to decrease bacterial load and rid wounds of necrotic tissue and debris. Once the wound is clean, balanced electrolyte or physiologic saline solutions are ideal for cleansing it (see Table 16-1). Tap water is not an ideal wound cleanser but is acceptable to initially remove dirt and debris when there is severe contamination. Tap water’s hypotonicity causes cell swelling, which can cause significant cell destruction and delay wound healing with prolonged use. Antiseptic solutions are used early in wound management to reduce bacterial numbers and reduce chances of infection. They are contraindicated in clean wounds because all antiseptics have some cytotoxic effects and may be more damaging than beneficial to wound healing.
Surgery of the Integumentary System
Wound Management
Wound Healing
Stages of Wound Healing
Host Factors Affecting Wound Healing
External Factors Affecting Wound Healing
Management of Open or Superficial Wounds
![]() TABLE 16-1
TABLE 16-1
CLEANSER
ADVANTAGE
DISADVANTAGE
Commercial cleansers:
Allclenz
Sur-Clenz
Cara-Klenz
Ultra-Klenz
Surfactant breaks the bonds between foreign bodies and the wound surface
Convenient
Most ionic surfactants and many nonionic surfactants have been shown to be toxic to cells, delay wound healing, and inhibit the wound’s defense mechanisms
Expense
Tap water
Availability
Inexpensive
Ease of application
Hypotonic
Cytotoxic trace elements
Not antimicrobial
Balanced electrolyte solution:
Lactated Ringer’s solution (LRS)
Normosol
Isotonic
Least cytotoxic
Not antimicrobial
Normal (0.9%) solution
Isotonic
Slightly more acidic than LRS
Not antimicrobial
0.05% Chlorhexidine
(1 part stock solution to 40 parts sterile water or LRS) or (~25 ml stock solution per liter)
Wide antimicrobial spectrum
Good residual activity
Not inactivated by organic matter
Precipitates in electrolyte solutions
More concentrated solutions are cytotoxic and may slow granulation tissue formation
Proteus, Pseudomonas, and Candida are resistant
Corneal toxicity
0.05% Chlorhexidine with Tris EDTA
Makes bacteria more susceptible to destruction by lysozymes, antiseptics, and antibiotics. Rapidly lyses P. aeruginosa, E. coli, and Proteus vulgaris
Increases antimicrobial effectiveness approximately 1000 fold.
Precipitates in electrolyte solutions
More concentrated solutions are cytotoxic and may slow granulation tissue formation
Corneal toxicity
0.1% povidone-iodine
(1 part stock to 100 parts LRS) or (~10 ml stock to 100 ml LRS)
Wide antimicrobial spectrum
Inactivated by organic matter
Limited residual activity
Cytotoxic at concentrations greater than 1%
Contact hypersensitivity
Thyroid disorders if absorbed
Débridement
Antibiotics
Topical Wound Medications
Topical Antimicrobials and Antibiotics
Topical Wound-Healing Enhancers
Wound Cleansing Solutions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Integumentary System