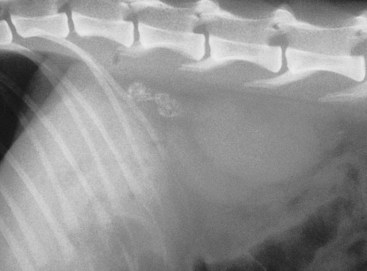
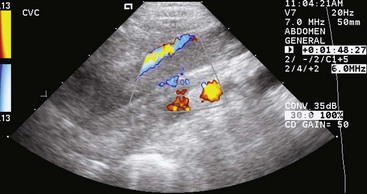
Chapter 23 Surgery of the Adrenal and Pituitary Glands Adrenocortical insufficiency may be primary, secondary to other diseases, or iatrogenic (i.e., due to administration of glucocorticoids, progestins, or drugs that suppress the adrenal glands [e.g., trilostane, mitotane, etomidate]). The history should include the dosages of corticosteroids or other drugs given, the type of corticosteroids, the duration of administration, and the time since the last dose. It is easier to inhibit secretion of glucocorticoids than mineralocorticoids (see the discussion of anatomy on p. 634). When glucocorticoid secretion is severely suppressed, the patient may experience depression, inappetence, lethargy, collapse, and/or weakness without electrolyte abnormalities. If mineralocorticoid secretion is suppressed, hyponatremia, hyperkalemia, acidosis, and/or azotemia may occur. Diminished ability to retain sodium results in volume depletion, diminished cardiac output, and reduced vascular tone, which may cause acute vascular collapse. Gastrointestinal disturbances and prolonged vomiting may contribute to electrolyte abnormalities and volume depletion. Electrolyte concentrations should be corrected before surgery. Some dogs with hypoadrenocorticism are hypoalbuminemic. A protective steroid release normally occurs during surgery that prevents circulatory collapse; however, animals with hypoadrenocorticism may be unable to respond appropriately and often require glucocorticoid supplementation before and during surgery. When minor elective surgery is performed in animals with adrenocortical insufficiency, glucocorticoid therapy may be given intravenously before induction of anesthesia (Box 23-1). The same dose can be given intravenously or intramuscularly after recovery from anesthesia, and the animal is returned to its oral maintenance glucocorticoid therapy the day after surgery. A similar protocol is used for major surgery, except that glucocorticoid therapy is continued at approximately five times the maintenance dose for 2 to 3 days (Box 23-2). Normal maintenance doses are then reinstituted. Once the animal is eating, medications can be given orally. A variety of anesthetic protocols may be used in adrenocortical-insufficient or hyperadrenal animals. Etomidate causes transient adrenal suppression and should be avoided in patients with hypoadrenocorticism and those in which postoperative hypoadrenocorticism is anticipated. Steroid replacement should be provided in animals showing signs of adrenal insufficiency. Maintenance of electrolyte and glucose concentrations is important. Glucocorticoid supplementation often is necessary in animals with adrenocortical insufficiency undergoing surgery (see previous discussion). Glucocorticoid therapy should be instituted before surgery in patients with HAC that are undergoing adrenalectomy. Because of the close association of the adrenals and the caudal vena cava, retraction of the caudal vena cava often is necessary for adrenalectomy. Vascular pressures should be closely monitored during surgery, and retraction should be done carefully to avoid obstructing venous return. Special anesthetic considerations are required for animals with pheochromocytomas to prevent complications associated with excessive secretion of catecholamines (see p. 643). The adrenal glands are near the craniomedial pole of the kidneys (Fig. 23-1). The left adrenal is slightly larger than the right. The left gland lies beneath the lateral process of the second lumbar vertebra; the right adrenal is more cranial, lying beneath the lateral process of the last thoracic vertebra. Because of the proximity of the right adrenal to the caudal vena cava, surgical removal of neoplastic glands can be difficult. The phrenicoabdominal (cranial abdominal) vessels cross the ventral surface of the adrenal. The adrenal glands are composed of two functionally and structurally different regions. The outer cortex produces mineralocorticoids (e.g., aldosterone), glucocorticoids, and small quantities of androgenic hormones. Mineralocorticoids regulate sodium and potassium concentrations. Aldosterone causes transport of sodium and potassium through the renal tubular walls and also causes hydrogen ion transport. Prepare the entire ventral abdomen and caudal thorax for aseptic surgery. Make a ventral midline abdominal incision that extends from the xiphoid cartilage to near the pubis. Identify the enlarged adrenal, and carefully inspect the entire abdomen, including the other adrenal, for abnormalities or evidence of metastasis. Palpate the liver for evidence of nodularity and biopsy if indicated. Palpate the caudal vena cava near the adrenals for evidence of tumor invasion or thrombosis. If additional exposure is necessary for adrenalectomy, extend the incision paracostally on the side of the affected gland by incising the fascia of the rectus abdominis muscle and the fibers of the external abdominal oblique, internal abdominal oblique, and transversus abdominis muscles, respectively. Use self-retaining retractors to improve visualization of the abdominal cavity. Retract the liver, spleen, and stomach cranially, the kidney caudally, and the vena cava medially to expose the entire gland. Identify the blood supply and ureter to the ipsilateral kidney, and avoid these structures during dissection. Ligate the phrenicoabdominal vein, and divide it between sutures. Using a combination of sharp and blunt dissection, carefully dissect the adrenal gland from surrounding tissue (Fig. 23-2). Numerous vessels may be encountered. Obtain hemostasis with electrocautery if the vessels are small or with hemoclips if they are large. If possible, do not invade the adrenal capsule. Removing the adrenal in one piece reduces the chances of leaving small pieces of neoplastic tissue in the abdominal cavity. If tumor thrombosis is present in the caudal vena cava but extensive metastasis is not apparent, temporarily occlude the vena cava using Rumel tourniquets (see p. 864). Make a longitudinal incision in the vein, and remove the thrombus. Close the vena cava in a continuous pattern with 5-0 or 6-0 vascular suture, and close the abdomen routinely (see discussion of suture material on p. 636). If a paracostal incision was made, begin the closure by approximating the abdominal wall at the junction of the combined ventral and paracostal incisions. After closing the linea alba, suture each muscle layer of the paracostal incision with a continuous pattern of synthetic absorbable sutures. Close the skin and subcutaneous tissue routinely. Place the patient in lateral recumbency with a rolled towel or a sandbag between the abdomen and the operating table. Prepare the caudal hemithorax and lateral abdomen for aseptic surgery. Make an incision just caudal to the thirteenth rib, extending it from the lateral vertebral processes to within 3 to 4 cm of the ventral midline (the incision will be approximately 10 to 14 cm long, depending on the animal’s size [Fig. 23-3]). Incise the abdominal muscles individually, and identify the adrenal gland cranial to the kidney. Retract the kidney ventrally, and ligate any vascular structures that cross its surface. Dissect the gland free from surrounding tissue (Fig. 23-4). Suture each muscle layer of the paracostal incision in a continuous suture pattern of synthetic absorbable (i.e., 2-0 or 3-0) material. Close the skin and subcutaneous tissues routinely. When no evidence of vena cava involvement is found, laparoscopic adrenalectomy may be an option (Pelaez et al, 2008; Mayhew, 2009). Laparoscopic adrenalectomy (LA) has been described in veterinary patients; however, the procedure is challenging and requires careful patient selection. Removal of the right adrenal gland in dogs is especially challenging because the gland capsule can be continuous with the tunica externa of the caudal vena cava. Preoperative diagnostic imaging is important in the work-up for adrenal masses and is the basis for deciding whether a laparoscopic approach might be feasible. The dimensions of the mass are vital, as are relationships to surrounding organs and vascular structures. Approximately 25% of adrenal neoplasms invade the vena cava, phrenicoabdominal veins, or renal vasculature; pheochromocytomas are more likely to invade than adrenocortical tumors. Vascular invasion is an indication for an “open” surgical approach. Ultrasonography and computed tomography are used most often for preoperative imaging. Ultrasonography has a sensitivity and a specificity of 80% and 90%, respectively, for detection of tumor thrombus. The sensitivity and specificity of CT or MRI for detection of vascular invasion are currently unknown, but recent experience with CT suggests that it is excellent for detection and delineation of tumor thrombus. Animals with functional tumors causing clinical signs and with those measuring more than 3 to 4 cm that do not exhibit vascular invasion should be considered for LA. Animals that are systemically unstable and those that have uncontrolled metabolic or acid-base disturbance, uncontrolled coagulopathies, untreated severe arrhythmias, or hypertension should not undergo LA. Animals that may be poorly tolerant of pneumoperitoneum (e.g., severe cardiorespiratory disease, diaphragmatic herniation) are poor candidates. Vascular invasion of the mass into surrounding vessels and large masses (>6 cm) are indications for open adrenalectomy; however, the effect of tumor size on morbidity during LA has not been evaluated in small animals. Inadequate training is an important contraindication for LA. For a description of LA, see the articles by Pelaez and associates (2008) and Mayhew (2009). After adrenalectomy, the patient’s hydration status and electrolyte balance should be monitored carefully and corrected as necessary. Bilateral adrenalectomy causes permanent adrenal insufficiency, and these animals require lifelong glucocorticoid (prednisone or prednisolone) and/or mineralocorticoid (deoxycorticosterone or fludrocortisone) replacement (Box 23-3). Animals should be closely monitored for hypoadrenocortical collapse. They are most likely to have an Addisonian crisis after they have been released to the owner’s care. Owners must be advised to watch for malaise, inappetence, vomiting, weakness, and other clinical signs suggesting decompensation. Temporary adrenal insufficiency occurs after unilateral removal of functional adrenal tumors because the tumor has suppressed the function of the contralateral adrenal. Glucocorticoids should be supplemented postoperatively (see Box 23-3) but may be discontinued when the remaining adrenal begins to function normally, as determined by the results of an ACTH stimulation test. Pulmonary thromboembolism is a potentially life-threatening complication of adrenal surgery, particularly in dogs with adrenal neoplasia. Sudden, severe postoperative respiratory distress may indicate pulmonary thromboembolism. Lung perfusion scans may help identify lung regions that are underperfused. Treatment with strict cage rest, oxygen, anticoagulants (e.g., aspirin, heparin), and thrombolytic agents (e.g., streptokinase) may be beneficial (see Box 23-3). Animals treated for pulmonary thromboembolism should be assessed frequently for signs of hemorrhage, and the hematocrit should be checked every 2 hours. If the packed cell volume (PCV) drops or hemorrhage is noted, the streptokinase infusion should be discontinued. The main complications of adrenalectomy are hemorrhage, fluid and electrolyte imbalance, pancreatitis, wound infection, delayed wound healing, and thromboembolism. Postoperative hemorrhage usually is associated with incomplete occlusion of small vessels surrounding an enlarged, highly vascular tumor. Judicious use of electrocautery and hemoclips helps prevent this complication. The Ligasure cautery device (Tyco Healthcare Group LP, Boulder, Colo.) has been useful for controlling hemorrhage from small vessels surrounding the adrenal gland during adrenalectomy. For prevention of thromboembolism, animals may be started on heparin during surgery and continued on it postoperatively (see Box 23-3). Delayed wound healing often is encountered in animals with HAC because of the adverse effect of steroids on wound healing; therefore, care should be taken with abdominal closure in these animals (see previous discussion). Strong monofilament absorbable (i.e., polydioxanone or polyglyconate) or nonabsorbable (i.e., polypropylene) suture should be used. Animals with adrenal neoplasia usually are old and often have concurrent abnormalities such as hypertension or cardiovascular abnormalities; therefore, extreme care should be exercised during anesthesia. These animals also require extensive postoperative monitoring. If the animal is debilitated, anorexic, or vomiting, placement of an enteral feeding tube during surgery (see p. 110) or parenteral nutrition (see p. 99) is advised. Mayhew, PD. Advanced laparoscopic procedures (hepatobiliary, endocrine) in dogs and cats. Vet Clin North Am Small Anim Pract. 2009;39:925. Pelaez, MJ, Bouvy, BM, Dupre, GP. Laparoscopic adrenalectomy for treatment of unilateral adrenocortical carcinomas: technique, complications, and results in seven dogs. Vet Surg. 2008;37:444. Specific Diseases Adrenal adenomas and carcinomas have been diagnosed in cats. Adrenal gland carcinomas in cats have been reported to secrete cortisol, progesterone, aldosterone, and testosterone (DeClue et al, 2005). Feline adrenal tumors may be unilateral or bilateral. Bilateral tumors may be of more than one tumor type. Progressive polyuria, polydipsia, polyphagia, aggression, and weight gain have been reported in a cat with adrenocortical adenoma in one adrenal gland and pheochromocytoma in the other (Calsyn et al, 2010). Primary hyperaldosteronism secondary to adrenal adenoma or carcinoma occurs in middle-aged and older cats with hypokalemic polymyopathy and/or systemic hypertension (Ash et al, 2005). Vomiting has been associated with intestinal perforation in a dog with adrenocortical adenoma, but this is rare. High circulating levels of glucocorticoids may make diagnosis of intestinal perforation difficult because signs of peritonitis (abdominal discomfort, restlessness, panting, weakness, and/or dyspnea) initially are obscured. Cats reported with primary hyperaldosteronism present with a history of vomiting, inappetence, dehydration, weakness, diarrhea, and cervical ventroflexion (Ash et al, 2005; Rose et al, 2007; Renschler and Dean, 2009). Spraying urine with a strong odor and aggressive behavior were included in the history of a cat with functional (elevated sex hormones androstenedione and testosterone) adrenal cortical adenoma (Millard et al, 2009). Clinical findings in animals with adrenocortical tumors depend on whether the tumors are functional. Dogs with ADH should have obvious signs of HAC (see previous discussion). Ascites, abdominal pain, edema, diarrhea, and vomiting are more common with nonfunctional tumors, although many are asymptomatic. Aggression has been associated with a cat with adrenocortical adenoma and pheochromocytoma (Millard et al, 2009; Calsyn et al, 2010) and blindness secondary to systemic hypertension (retinal detachment) and weakness may be seen in cats with primary aldosteronism (Ash et al, 2005). Adrenal tumors are difficult to detect radiographically unless they are associated with significant adrenal enlargement (20 mm or larger) or calcification. Food should be withheld for 24 hours before radiography to allow the gastrointestinal tract to empty. In some dogs, mineralization of tissue cranial to the kidney may be seen on survey radiographs and may or may not be associated with obvious adrenal enlargement. This finding is suggestive of adrenocortical neoplasia (adenoma or carcinoma). Nonneoplastic mineralization of adrenal glands is rare in dogs; however, bilateral adrenal calcification may occur with PDH. Conversely, adrenal gland mineralization is considered an incidental finding in cats (Fig. 23-5). Hepatomegaly, calcinosis cutis, or osteoporosis may be seen with PDH and ADH. Enhanced abdominal contrast caused by increased abdominal fat may occur. Dogs with HAC are more likely to have calcium-containing uroliths than dogs without clinical evidence of HAC. Although pheochromocytomas may be detected radiographically if sufficiently large, ultrasonography is more sensitive. CT and MRI allow accurate localization of adrenal neoplasia, but they do not allow differentiation of tumor type. Adrenal carcinomas may appear as well-demarcated, homogeneous masses, or they may be poorly demarcated, with irregular texture and contrast enhancement. Masses that are poorly demarcated, irregularly shaped, and nonhomogeneous with mineralization usually are carcinomas. Ultrasound can detect caudal vena cava invasion, but contrast-enhanced CT is more sensitive. Administration of contrast agents in patients with pheochromocytoma may produce severe hypertension; thus, it should be done cautiously. If contrast-enhanced CT and ultrasound are not performed, caudal vena caval angiography (note precautions in previous discussion) may be considered before surgery if caudal vena caval thrombosis is suspected (Fig. 23-6). Excretory urography may help identify tumor invasion requiring nephrectomy (i.e., ureteral obstruction or renal invasion). Diagnosis of HAC requires clinical signs consistent with the disease. Abnormal adrenal function test results in the absence of clinical signs are not diagnostic of HAC. The ACTH stimulation test (Box 23-4) and the low-dose dexamethasone suppression (LDDS) test are the primary adrenal function tests used. The ACTH stimulation test is easy and quick to perform, and is the best test to look for iatrogenic HAC (Box 23-5). However, the ACTH stimulation test has disadvantages. Dogs with ADH can have almost any response (i.e., normal, exaggerated, or diminished) to ACTH, and many dogs without HAC have exaggerated test results that mimic HAC. Classically, one measures cortisol concentrations before and after administration of ACTH, but one may also measure sex steroids (e.g., 17-hydroxyprogesterone). However, the sensitivity and specificity of measuring sex steroids are still being determined at the time of this writing, and some evidence suggests that increased 17-hydroxyprogesterone concentrations post ACTH administration may not be as specific for HAC as was originally thought. The LDDS test requires 8 hours to perform, but it is a better test to look for spontaneous HAC. Furthermore, the LDDS test often permits differentiation of PDH and ADH. Functional adrenocortical carcinoma may cause decreased aldosterone concentrations and increased deoxycorticosterone concentrations in the presence of hypokalemia. These metabolic abnormalities have been shown to resolve with resection of the carcinoma (Davies et al, 2008). Elevated serum aldosterone levels have been found in all cats with hyperaldosteronism secondary to adrenal tumor (Ash et al, 2005; Rose et al, 2007; Renschler and Dean, 2009). Other laboratory findings include hypokalemia, a possibly increased Na : K ratio, systemic hypertension, hyperglycemia, hypophosphatemia, and creatine kinase. The low-dose dexamethasone suppression test was used to diagnose hyperadrenocorticism in a cat with bilateral adrenomegaly. Surgical resection resulted in diagnosis of cortical adenoma in the right and pheochromocytoma in the left adrenal gland (Calsyn et al, 2010). Adrenal gland tumors in cats can produce a variety of hormones other than cortisol. Sex hormonal analyses in a cat revealed increases in serum concentrations of androstenedione and testosterone associated with a functional adrenal cortical adenoma (Millard et al, 2009). Laboratory abnormalities are inconsistent and nonspecific in animals with pheochromocytomas. Adrenergic blockage (e.g., phenoxybenzamine, phentolamine, prazosin) is used to control blood pressure in patients with pheochromocytoma. These drugs are also used preoperatively and intraoperatively (see discussions of Preoperative Management and Anesthesia on pp. 642 and 643, respectively). If tachycardia or cardiac arrhythmias are present, β-adrenergic blockade may be used; however, unopposed β-blockade may cause severe, life-threatening hypertension. β-Blockade should not be started until α-adrenergic blockade has been determined to be adequate. In one study, phenoxybenzamine-treated dogs undergoing adrenalectomy for pheochromocytoma had a significantly decreased mortality rate compared with untreated dogs (13% vs. 48%, respectively) (Herrera et al, 2008). Mitotane (o,p′-DDD) destroys the adrenal cortex in a dose-dependent fashion. It was the major drug used to treat canine HAC before the advent of trilostane. Mitotane can often control clinical signs in animals with ADH; however, tumors are more resistant to the adrenocorticolytic effects of mitotane than are normal or hyperplastic adrenal cortices. Larger doses (Box 23-6) are often required to attain and maintain control in ADH dogs than in PDH dogs, and stronger side effects (e.g., gastric irritation, vomiting) can be expected. Major advantages of mitotane include the following: (1) it is effective, and (2) after an induction therapy (usually 4 to 14 days), maintenance therapy consists of one to two treatments per week. Major disadvantages are that (1) it can easily destroy the entire adrenal gland, resulting in temporary or permanent hypoadrenocorticism (or death), (2) some dogs are resistant to its effects, and (3) some dogs are very sensitive to its effects. The last two disadvantages mean that there is substantial variation in how patients respond. Some patients respond well and are easy to treat, whereas others are extremely difficult to control using mitotane. This is why trilostane has become the more popular treatment for HAC. Ketoconazole causes reversible inhibition of adrenal steroid production and has little effect on mineralocorticoid production. Therefore, ketoconazole (Box 23-7) may be used (1) in dogs with ADH who are not surgical candidates, (2) before surgery to reduce anesthetic and surgical risks in animals with uncontrolled HAC, and (3) as a diagnostic trial in dogs in which equivocal test results make the diagnosis of HAC difficult. If used for diagnostic purposes, the drug should be given for a minimum of 4 to 8 weeks. Major advantages of ketoconazole include the following: (1) it is relatively safe, and (2) it is very effective. Major disadvantages are that (1) it works only while there are blood levels of drugs, (2) it is relatively expensive, and (3) it is so effective at decreasing cortisol concentrations that it is easy to make patients feel sick when first starting the drug. Furthermore, adverse reactions to ketoconazole (e.g., anorexia, depression, vomiting, diarrhea, icterus) may occur, necessitating that the drug be dropped or the dosage reduced. If an overdose is suspected of causing acute illness or collapse, one should administer glucocorticoids and stop the ketoconazole. It is seldom used for HAC since the advent of trilostane.
Surgery of the Endocrine System
General Principles and Techniques
Preoperative Management
Anesthesia
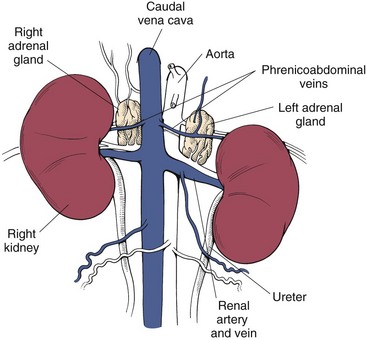
Surgical Anatomy
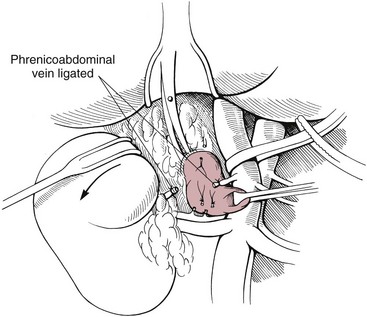
Surgical Technique
Adrenalectomy via a Midline Abdominal Approach
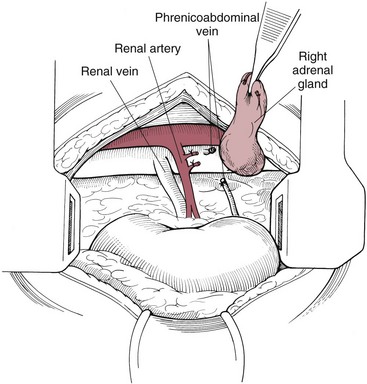
Adrenalectomy via a Paralumbar Approach
Laparoscopic Adrenalectomy
Postoperative Care and Assessment
Complications
Special Age Considerations
References
Adrenal Neoplasia
General Considerations and Clinically Relevant Pathophysiology
Diagnosis
History
Physical Examination Findings
Diagnostic Imaging
Laboratory Findings
Medical Management