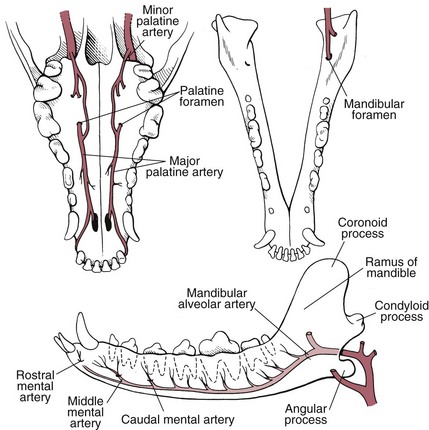
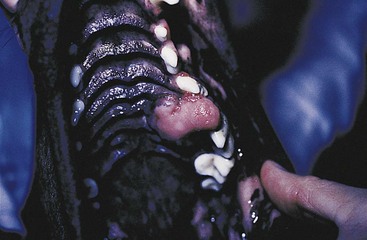
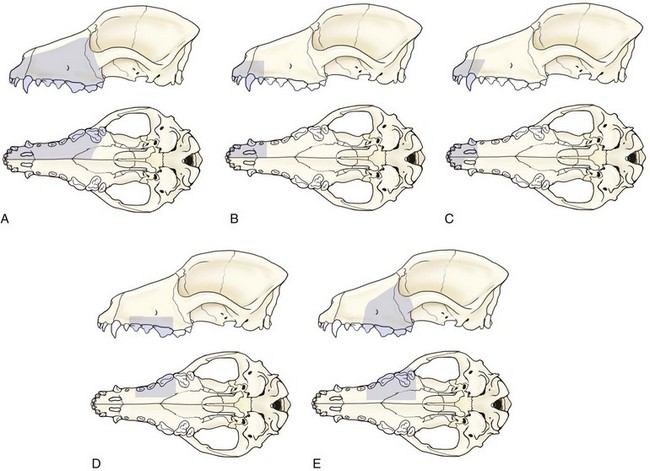
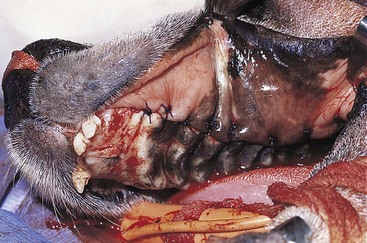
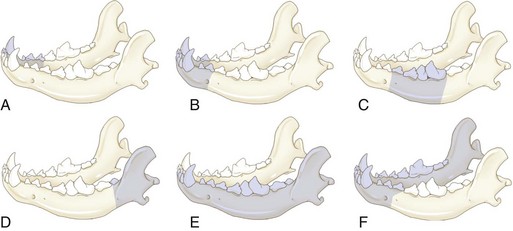
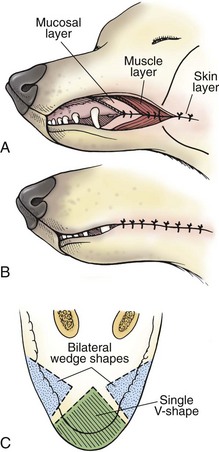
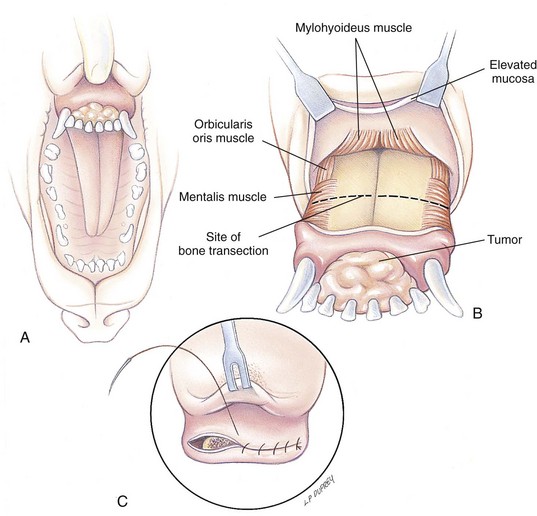
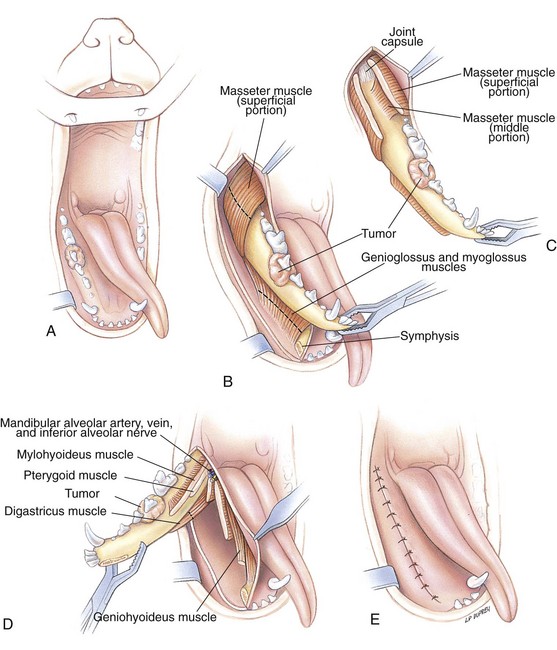
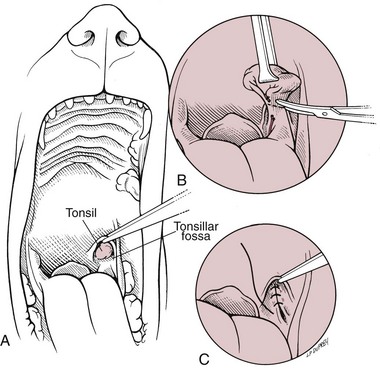
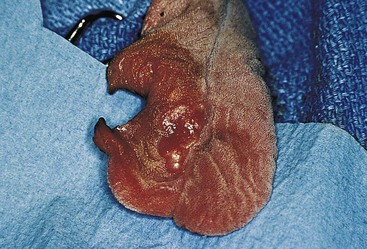
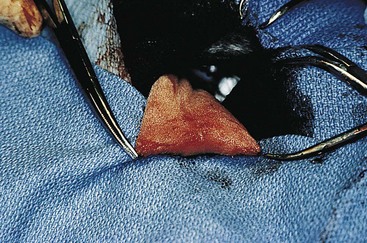
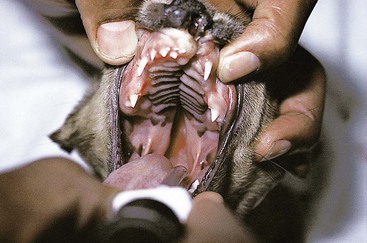
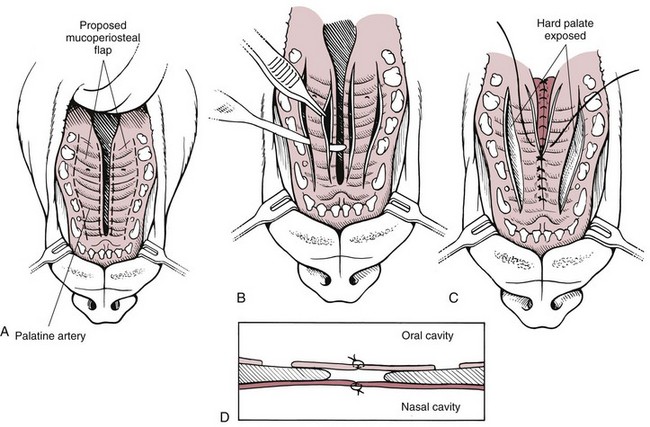
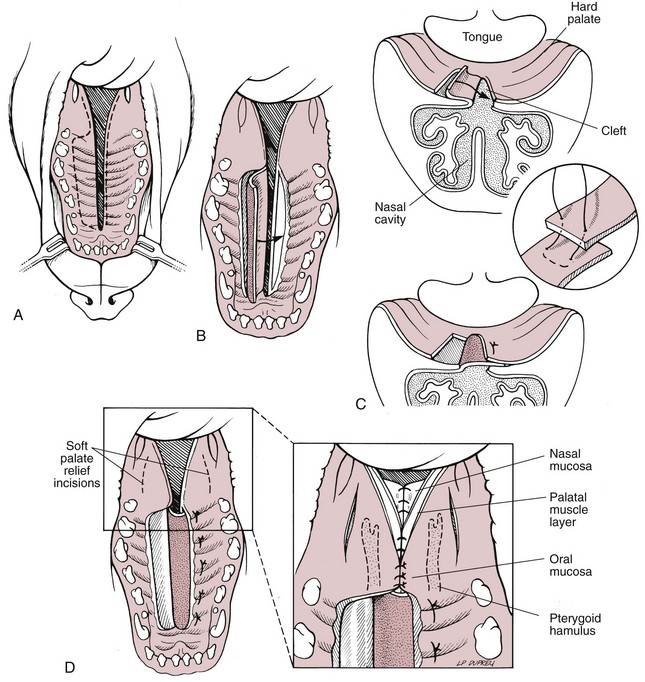
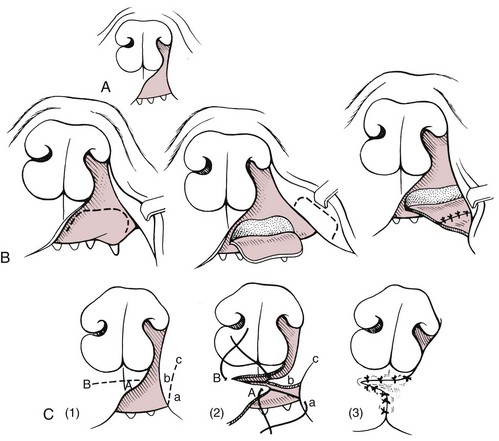
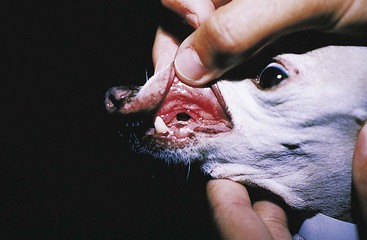
Chapter 20 Surgery of the Oral Cavity and Oropharynx Surgical diseases of the oral cavity and oropharynx are common in dogs and cats. They include congenital and traumatic abnormalities, foreign bodies, neoplasia, salivary gland disease, and dental disease. Patients with oral cavity or oropharyngeal disease may have drooling, dysphagia, anorexia, bleeding from the mouth, and/or fetid breath. Some animals are asymptomatic until the lesions become large or are discovered on routine physical examination. Others are presented for treatment of a mass, oral hemorrhage, oral pain, difficulty eating, nasal regurgitation, chronic rhinitis, dyspnea, or all of these. Oropharyngeal disorders often present with a combination of upper airway and upper gastrointestinal tract signs including cough, sneezing, dysphagia, and dyspnea if the laryngopharynx is involved (Billen et al, 2006). They may have a history of dental disease, weight loss, or trauma. The diagnosis is based on history, clinical signs, physical examination, cytologic studies, radiographs, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and/or biopsy. Before major surgery is performed, a thorough physical examination, complete blood cell count (CBC), and serum biochemical profile should be performed; urinalysis and electrocardiogram (ECG) may be appropriate too. Animals undergoing maxillectomy or mandibulectomy and those predisposed to coagulopathies should have the coagulation system checked (i.e., platelet count and mucosal bleeding time) and blood cross-matching done before surgery. Skull radiography, MRI, or CT images usually can determine the extent of the lesion. Thoracic radiographs are indicated to evaluate for metastasis, cardiac size, and pulmonary disease. The teeth of animals with periodontal disease should be cleaned several days before major reconstructive surgery to improve tissue health and reduce oral bacterial numbers. Nutrition should be maintained by tube feeding if necessary (see Chapter 10). Animals with oronasal fistulae can be fed via feeding tubes to reduce the chances of rhinitis and inhalation pneumonia before surgery. Metabolic abnormalities should be corrected. Mature animals should be fasted for 12 to 18 hours before induction of anesthesia (pediatric patients are fasted 4 to 8 hours). After induction and orotracheal intubation, the mouth should be flushed with dilute Betadine or chlorhexidine solution to reduce bacterial numbers. Preemptive analgesics and local nerve blocks (infraorbital, maxillary, mandibular, mental, or palatine nerves; bupivacaine 0.5%, 0.25 to 1 ml per site; see also Chapter 12) should be used to minimize pain associated with major oral reconstructive surgery. An orally placed endotracheal tube can sometimes hinder oral cavity and oropharyngeal surgery; in these cases, endotracheal intubation can be performed through a pharyngotomy (see p. 101) or tracheotomy incision (see p. 916). It is important that the endotracheal tube and its cuff prevent blood and fluid from entering the lower airways. One or two gauze sponges can be placed in the oropharynx around the endotracheal tube to help absorb fluids; however, be sure to remove these sponges prior to waking the patient. Postoperative swelling of the oral mucous membranes may obstruct the glottis; this can be minimized by pretreatment with corticosteroids (e.g., dexamethasone, 0.5 to 1 mg/kg given subcutaneously or intramuscularly before induction of anesthesia or intravenously at induction). Most animals with oral disease are healthy, and numerous anesthetic protocols can be used (Table 20-1). Because the oral cavity is highly vascular and hemostasis can sometimes be difficult, blood, hypertonic saline, and/or colloids should be available in the event of severe hemorrhage (see p. 33). Preoperative placement of two large cephalic catheters provides better venous access if volume replacement becomes necessary. Evaluation of blood pressure during surgery is warranted because of the risk of bleeding and subsequent hypotension. Anesthetic Considerations in the Healthy Patient Undergoing Oral or Rectal Surgery *Monitor for hyperthermia in cats. †Buprenorphine is a better analgesic than morphine in cats. ‡Use only in young, healthy animals. §Don’t exceed maximum local anesthetic dose for very small dogs and cats. The oral cavity and oropharynx are contaminated (aerobic, facultative, and anaerobic bacteria); but saliva is antimicrobial, and blood supply to this region is excellent. For these reasons, infections after oral surgery are rare. One dose of a prophylactic antibiotic effective against Gram-positive aerobes and anaerobes (e.g., ampicillin, clindamycin) may be given at anesthetic induction (Box 20-1). Therapeutic antibiotics (e.g., cefazolin plus metronidazole, amoxicillin plus clavulanic acid, or clindamycin; see Box 20-1) are indicated in debilitated or immunosuppressed patients and those with severe periodontal disease. The blood supply to this region originates from branches of the common carotid arteries. The paired major and minor palatine arteries are important (Fig. 20-1). Two or three vessels emerge from the major palatine foramen at the caudal edge of the fourth upper premolar and course rostrally, midway between the midline and the dental arcade. The right and left major palatine arteries anastomose caudal to the incisors. The minor palatine arteries enter the palate caudal to the last molar and lateral to the major palatine artery, then course caudomedially to ramify in the caudal hard palate and soft palate. The major blood supply to the mandible is via the mandibular alveolar artery, which enters the mandibular canal on the medial surface of the mandible (see Fig. 20-1). The entry point is where an oblique line connecting the last molar tooth and the angular (muscular) process (which is hidden beneath the pterygoid muscle) meet. The mandibular alveolar artery ends at the middle mental foramen, where it branches to form the caudal, middle, and rostral mental arteries and exits via the mental foramina. The mandibular canal also transmits the mandibular vein and the mandibular alveolar nerve. Atraumatic surgical technique is important for reducing tissue damage and swelling and encouraging rapid healing (Box 20-2). Hemorrhage is expected and should be controlled with pressure and vessel ligation. Electrosurgery should be used sparingly because excessive use delays healing and may cause dehiscence. Electrocoagulation should be applied only to discrete, isolated areas. The possibility exists that flammable gases (i.e., oxygen) leaking around the endotracheal tube could be ignited when electrosurgery or laser surgery is used. Some surgeons perform temporary carotid artery occlusion before maxillectomy to minimize blood loss. Maxillectomy is most often performed to resect an oral neoplasm (Fig. 20-2). Varying amounts of the maxilla and hard palate may be excised, depending on the gross and radiographic, CT, or MRI extent of the lesion. Depending on the area being resected, partial maxillectomies may be classified as hemimaxillectomies (rostral, central, or caudal) or premaxillectomies (bilateral rostral) (Figs. 20-3 and 20-4). Hemimaxillectomy, without a definition of site, usually refers to removal of one entire maxilla. Partial maxillectomies can be combined with nasal planectomy and dorsolateral approaches through the skin (Lascelles et al, 2004). Partial maxillectomy is limited by the surgeon’s ability to reconstruct the oronasal defect; lesions that cross the midline of the palate are difficult to reconstruct. Use an oscillating saw or an osteotome and mallet to cut the maxilla, incisive bone, and/or palate. Resect all premolar and molar teeth for lesions extending to the third premolar because of the outward turn of the dental arch. When performing a caudal maxillectomy, remove a portion of the zygomatic arch and orbit if necessary to obtain clean borders. Elevate the tissue block and sever any remaining soft tissue attachments to complete the resection and expose the nasal cavity (Fig. 20-5). Remove involved nasal turbinates with rongeurs and hemostats if disease extends into the nasal cavity. Control hemorrhage by ligating identifiable vessels and applying pressure to other areas. Isolate and ligate the major palatine and infraorbital artery and vein if they are included in the resection site. Use bone wax or electrofulguration to help control bone hemorrhage. Lavage and inspect the defect to ensure that all grossly diseased tissue has been excised. Close the defect by elevating a buccal mucosal flap from the adjacent cheek or lip (Fig. 20-6). Elevate enough buccal mucosa and submucosa to allow a tension-free approximation with the gingival and palatal mucosa. Place the first layer of simple interrupted sutures in the submucosa with the knot directed toward the nasal cavity. Place a second layer of interrupted approximating sutures (i.e., simple, cruciate, vertical mattress) to accurately appose buccal mucosa to the palatal and gingival mucosa. A double-flap technique may be used to close premaxillectomy defects to provide mucosa on both the nasal and oral surfaces. However, an epithelial surface on the nasal aspect of the flap is not necessary because the connective tissue surface of the flap is covered with respiratory epithelium within 1 to 2 weeks. If carotid artery occlusion was performed, release the occlusion after the defect has been closed. FIG 20-5 A central hemimaxillectomy was performed to remove the lesion shown in Fig. 20-2 and an additional 1 to 2 cm of normal tissue. Note that the nasal cavity is exposed. FIG 20-6 A buccal mucosal flap was advanced over the defect evident in Fig. 20-5 and secured with approximating sutures. Mandibulectomy is most often performed to resect an oral neoplasm. Occasionally mandibular fractures are also treated by partial mandibulectomy. Varying amounts of mandible may be excised, depending on the extent of the tumor or lesion (Fig. 20-7). Depending on the extent of resection, hemimandibulectomies may be classified as rostral, rostral-bilateral, central, caudal, or total (Fig. 20-8). These techniques may be combined when more extensive resection is necessary. After mandibulectomy, cheiloplasty (commissuroplasty) may be performed to minimize excessive drooling and lateral protrusion of the tongue (Fig. 20-9). It is accomplished by removing the mucocutaneous junction of the upper and lower lip to the level of the second premolar or canine tooth. The commissure is advanced rostrally during closure. The upper and lower lip margins are apposed in three layers (oral mucosa, muscle and connective tissue, and skin) (Fig. 20-10). Opening the mouth fully during the first 2 weeks may cause dehiscence. Tension-relieving button sutures or a loose tape muzzle may be used to help prevent this. During rostral mandibulectomies, redundant skin and mucosa may be eliminated by excising and apposing V-shaped wedges. The base of the V is along the mucocutaneous junction (see Fig. 20-10). Position the patient in lateral, sternal, or dorsal recumbency with the neck extended (Figs. 20-11, A, and 20-12, A). Clip and aseptically prepare the skin of the lateral face and ventral mandible. Flush the mouth with antiseptic solution. Determine the amount to be resected based on the size of the soft tissue lesion and the radiographic, CT, or MRI evidence of bony involvement. Generally, excise the mass and a minimum of 1 to 2 cm of normal soft tissue and bone on all borders. Retract the commissure and lip to give maximum exposure. If necessary, improve visualization by incising the commissure to the level of the mandibular angle (Fig. 20-12, B). Begin en bloc resection by first incising mucosa (buccal, gingival, and sublingual) around the diseased area (Figs. 20-11, B, and 20-12, B). Using a periosteal elevator, undermine and reflect the gingival mucosa to expose the lateral and ventral aspects of the ramus. Transect or elevate and retract muscles (mentalis, orbicularis oris, buccinator, mylohyoideus, geniohyoideus, genioglossus, masseter, digastricus, temporalis, and pterygoideus) attached to the portion of the mandible being resected (Figs. 20-11, B, and 20-12, C and D). Use an oscillating saw or an osteotome and mallet to transect the ramus and separate the symphysis. As an alternative, use a Gigli wire to transect the ramus. Complete a total hemimandibulectomy by incising the joint capsule and disarticulating the temporomandibular joint (Fig. 20-12, C). Locate the temporomandibular joint by rotating the mandible and palpating the articulation. Ligate or cauterize the mandibular artery (see Fig. 20-12, D). Sever any remaining soft tissue attachments to complete the resection. Avoid traumatizing the lingual frenulum or sublingual and mandibular salivary ducts. Contour the ostectomy sites with bone rongeurs, removing sharp bone and tapering the edges to facilitate closure. Close the defect by elevating a mucosal flap from the adjacent lip or cheek (Figs. 20-11, C, and 20-12, E). Elevate enough mucosa and submucosa to allow a tension-free approximation with the gingival and sublingual mucosa. Place the first layer of simple interrupted sutures in the submucosa with the knots buried. Place a second layer of interrupted approximating sutures (simple, cruciate, or vertical mattress) to accurately appose the labial, sublingual, and gingival mucosa. As an alternative, use a single-layer, simple continuous or interrupted suture pattern. Mandibular rim excision may be considered for small, minimally invasive tumors of the mandible in large dogs in which the tooth roots do not approach the mandibular canal (Arzi and Verstraete, 2010). Advanced imaging is strongly recommended to help ensure complete excision of mass lesions using this technique. Incise the mucoperiosteum in a curvilinear pattern 1 cm around the mass and associated teeth. Elevate the attached gingiva and excise the mandibular segment with a handpiece that irrigates but does not insufflate. Be certain that no tooth roots remain. Smooth the osteotomy site, and reattach the mucosa over the bony defect with monofilament absorbable suture. Administer dexamethasone (0.1 to 0.2 mg/kg IV) at the time of induction to minimize postoperative swelling and edema. Position the animal in ventral recumbency with the maxilla suspended from an IV stand or similar device. Open the mouth maximally, and secure it open with tape or gauze. Locate the tonsil in the tonsillar fossa or crypt on the dorsolateral wall of the oropharynx just caudal to the palatoglossal arch (Fig. 20-13). Retract the edge of the tonsillar crypt caudodorsally to expose the tonsil. Grasp the tonsil at its base with an Allis tissue forceps or hemostat and retract it from the crypt. Transect the hilar mucosa at the base of the tonsil with Metzenbaum scissors or a tonsillectomy snare. Ligate the tonsillar artery as it enters the caudal aspect of the tonsil. Some surgeons excise the tonsil using electrosurgery or laser surgery. Appose the edges of the tonsillar crypt with a simple continuous suture pattern of 3-0 or 4-0 monofilament absorbable suture to minimize hemorrhage. The primary reason for partial tongue amputation is neoplasia, which usually occurs on the margin or base of the tongue. The most common tongue tumor is squamous cell carcinoma (Fig. 20-14); others include malignant melanoma, granular cell myeloblastoma, and mast cell tumor. Most lacerations of the tongue are amenable to repair with a one-layer or two-layer closure rather than amputation. Other reasons for tongue surgery include abscess drainage, glossitis, severe trauma, and congenital ankyloglossia (limited tongue movement). Tongue lesions are associated with signs of drooling, malodor, dysphagia, and sometimes dyspnea. Glossectomies are classified as partial (involving only the free tongue), subtotal (entire free tongue and part of the genioglossus and geniohyoideus muscles), near total (greater than 75% of tongue), and total (100% of tongue) glossectomies. Glossectomy is considered major if more than the free portion of the tongue is removed. Amputation of 40% to 60% of the rostral tongue usually is well tolerated. Amputation at the base of the tongue or total glossectomy makes eating and drinking difficult; however, the animal can learn to suck in food and water, or chunks of food can be tossed to the base of the tongue. Ptyalism following major glossectomy occasionally occurs; improvement may be seen with bilateral sialadenectomy. When performing glossectomy, resect the diseased portion of the tongue and a minimum of 2 cm of normal tissue after placing a noncrushing clamp across the base of the tongue. When total glossectomy is performed a clamp is not used. Wedge the incision so that slightly more tongue muscle than dorsal or ventral mucosa is excised. Control hemorrhage by ligation, pressure, or electrosurgery. Use through-and-through horizontal mattress sutures as needed to control hemorrhage. Appose the epithelial edges with a simple continuous suture pattern using 3-0 or 4-0 monofilament absorbable suture (Fig. 20-15). Place a feeding tube when major glossectomies are performed (see pp. 101-110). Pharyngotomy is performed to allow endotracheal intubation or tube feeding. For a description of the technique, please see page 101 and Figs. 10-4 and 10-5. The salivary glands most often are removed to treat salivary mucoceles or neoplasia, and the mandibular and sublingual salivary glands are most often removed to treat cervical, sublingual, and pharyngeal salivary mucoceles. Neoplasms (usually adenocarcinomas or carcinomas) occur most frequently in parotid (50% dogs, 19% cats) and the mandibular (30% dogs, 59% cats) salivary glands (Hammer et al, 2001). Salivary gland excision is described on pp. 419-421. After oral surgery, gauze sponges should be removed from the caudal pharynx, and the nasopharynx should be suctioned. Extubation should be delayed until a well-developed swallowing reflex is present. Patients should be recovered in a slightly head down position, and the tube should be removed with the cuff slightly inflated to help ensure blood clots are expelled through the mouth rather being aspirated or swallowed. These patients should be monitored for airway obstruction or pain, and analgesics should be provided as needed (for opioid analgesics, see Table 12-3 on p. 141; for nonsteroidal anti-inflammatory drugs (NSAIDs), see Table 12-6 on p. 152). Elizabethan collars or similar restraining devices should be used on some animals to prevent disruption of the surgical site. Occasionally an acrylic oral splint is used to protect the surgical site. Oral intake should not be allowed for the first 8 to 12 hours after surgery (except in pediatric patients at risk for hypoglycemia); hydration should be maintained with intravenous fluids. Water should be offered after 12 hours and the animal observed for signs of dysphagia, pain, or regurgitation. If no serious problems are identified, soft food may be offered between 12 and 24 hours after surgery. Gruel is unnecessary and may seep between sutures, inhibiting healing. Hand feeding (tossing meatballs) and watering may be necessary for 1 to 2 weeks following major glossectomy, until the animal is trained to suck water and prehend food without a tongue. Feeding through a gastrostomy, pharyngostomy, or esophagostomy tube occasionally is necessary for animals with severe wounds or those unwilling to eat within 3 days after surgery (see Chapter 10). Soft food should be fed until the wound has healed and the animal prevented from chewing on sticks, toys, or other hard surfaces. Healing should be evaluated at 3 to 5 days, 2 weeks, and 4 weeks after surgery. Additional reconstruction may be necessary if areas of partial dehiscence do not heal by second intention or if an oronasal fistula remains after 4 to 6 weeks. Animals with neoplasia should be evaluated every 3 to 6 months for tumor recurrence. Cosmesis and function after partial mandibulectomy are good (see Fig. 20-7). Mandibular “drift” and instability occur more often when the osteotomy is caudal to the second premolar. The remaining hemimandible may deviate medially, producing malocclusion, clicking of the teeth, and/or trauma to the palatal or gingival mucosa. Most animals adapt, and serious problems seldom occur. The involved canine tooth may be pulled or shortened if erosion or ulceration develops. Alternatively, use of orthodontic elastic chain attached to the ipsilateral canine and maxillary fourth premolar has been shown to decrease mandibular drift in a small number of patients. Significant client compliance is required in changing the chain weekly for 4 to 6 weeks, but indefinite use has been considered for prevention of mandibular drift (Bar-Am and Verstraete, 2010). Rostral mandibulectomy (bilateral) caudal to the third or fourth premolar may cause difficulty with prehension and is less cosmetic. Tongue protrusion may occur, but most patients are able to keep the tongue retracted. Cheiloplasty may decrease drooling, and lateral tongue protrusion in animals after partial mandibulectomies or maxillectomies. Minor swelling of the skin and mucous membranes should be expected after partial maxillectomy or mandibulectomy but should resolve within 2 to 3 days. Infection is possible because the oral cavity is contaminated. However, infection is rare if the blood supply is maintained and good surgical technique is used. Partial dehiscence may occur 3 to 5 days after surgery if tissue is severely traumatized, blood supply is inadequate, or excessive motion or tension affects any area of the repair. Sometimes appearances that are unacceptable to owners may be modified with further cosmetic reconstruction following maxillectomy or mandibulectomy. Zygomatic mucocele has been reported after caudal hemimaxillectomy in a dog (Clarke and L’Eplattenier, 2010). The most common postoperative complication after tonsillectomy is hemorrhage. Regrowth of tonsillar tissue may occur if excision is incomplete. After pharyngotomy, complications of airway obstruction and aspiration pneumonia may be seen if the pharyngostomy tube is located cranial or ventral to the epihyoid bone. Apparent hyperptyalism following major glossectomy occasionally occurs; improvement may be seen with bilateral sialadenectomy. Tumor recurrence or metastasis is always possible after any oncologic surgery. Pediatric patients with congenital cleft palate should be fed with a tube until they are 8 to 12 weeks old and may be more safely anesthetized. Refer to the anesthesia section in Chapter 27 (see p. 783) for discussion of special concerns with pediatric patients. Pediatric patients are at higher risk of hypothermia and hypoglycemia; therefore, they should not be fasted for more than 4 to 8 hours. Neoplasia is more common in geriatric patients; they should be thoroughly evaluated before surgery for concurrent disease and metastasis. Arzi, B, Verstraete, FJ. Mandibular rim excision in seven dogs. Vet Surg. 2010;39:226. Bar-Am, Y, Verstraete, FJ. Elastic training for the prevention of mandibular drift following mandibulectomy in dogs: 18 cases (2005-2008). Vet Surg. 2010;39:574. Billen, F, Day, MJ, Clercx, C. Diagnosis of pharyngeal disorders in dogs: a retrospective study of 67 cases. J Small Anim Pract. 2006;47:122. Clarke, BS, L’Eplattenier, HF. Zygomatic mucocele as a postoperative complication following caudal hemimaxillectomy in a dog. J Small Anim Pract. 2010;51:495. Hammer, A, Getzy, D, Ogilvie, G, et al. Salivary gland neoplasia in the dog and cat: survival times and prognostic factors. J Am Anim Hosp Assoc. 2001;37:478. Lascelles, BD, Henderson, RA, Seguin, B, et al. Bilateral rostral maxillectomy and nasal planectomy for large rostral maxillofacial neoplasms in six dogs and one cat. J Am Anim Hosp Assoc. 2004;40:137. Specific Diseases Congenital palatal defects result when the two palatine shelves fail to fuse during fetal development (Fig. 20-16). The most critical time for development and closure of the fetal palate appears to be 25 to 28 days of gestation in dogs. Incomplete closure of either the primary or secondary palate is attributed to inherited (recessive or irregular dominant, polygenic traits), nutritional (inadequate folic acid), hormonal (steroids), mechanical (in utero trauma), and toxic (including viral) factors. Primary cleft palate alone is rare (Fig. 20-17); however, secondary cleft palate may occur alone or in combination with primary clefts. Some affected neonates are unable to nurse effectively and die soon after birth. Others contaminate their nasal cavity with saliva and food. Signs of rhinitis and other respiratory infections are common. Middle ear disease that is often clinically unrecognized is sometimes associated with congenital clefts. Affected patients should be tube fed to maintain an adequate nutritional status and reduce the incidence of aspiration pneumonia until they are old enough for surgery. Aspiration pneumonia may be treated with antibiotics, fluids, oxygen, bronchodilators, and/or expectorants. Use of corticosteroids for acute aspiration is controversial and clearly has no place in chronic aspiration. A tracheal wash or brushing for culture and sensitivity testing should be performed if aspiration pneumonia is severe. Broad-spectrum antibiotics with efficacy against anaerobes (Box 20-3) are indicated for severe aspiration or purulent rhinitis. Animals with severe rhinitis may benefit from having the nasal infection treated before surgical closure of the defect. Cultures should be obtained from the nasal cavity after anesthetizing the patient and the nose and oral cavity flushed of debris and exudate. To prevent recontamination of the nasal cavity with food, the animal should be given nothing by mouth for 10 to 14 days. Nutrition can be provided via tube feeding (e.g., esophagostomy or gastrostomy tube; see pp. 102 and 106, respectively) during this time. Puppies and kittens are better able to metabolize drugs after 8 weeks of age, making them better anesthetic risks. Special precautions should be taken to prevent hypothermia and hypoglycemia in young patients. Refer to the anesthesia section in Chapter 27 (see p. 783) for discussion of special concerns with pediatric patients. Intubation through a pharyngotomy (see p. 101) or tracheotomy incision (see p. 916) may facilitate repair of secondary clefts. Pharyngotomy generally is preferred if it allows adequate visualization of the defect. General anesthetic recommendations for animals undergoing oral surgery are provided on page 386. Guarded tracheostomy tubes should be used to prevent kinking during the procedure. Care should be taken to prevent and recognize dislodgment of the anesthetic tubing from the endotracheal tube during oral manipulations. The major palatine arteries emerge from the major palatine foramen midway between the midline and caudal edge of the upper fourth premolar (see Fig. 20-1). The main artery courses rostrally equidistant between the lingual border of the teeth and the palatal midline to anastomose with the major palatine artery of the contralateral side caudal to the incisors. The minor palatine arteries enter the palate at the level of the last molar, caudal and slightly lateral to the major palatine foramen. The minor palatine arteries course caudomedially and ramify in the caudal hard palate and soft palate. The soft palate is also supplied by branches of the ascending pharyngeal artery. Incise the margins of the defect and make bilateral releasing incisions along the margins of the dental arcade (Fig. 20-18, A). Elevate the mucoperiosteal layer on both sides of the defect with a periosteal elevator (Fig. 20-18, B). Avoid damaging the major palatine arteries. Control hemorrhage with pressure and suction. Appose the nasal mucosal edges or periosteum at the margin of the defect with buried interrupted sutures (knots within the nasal cavity) if possible. Slide the elevated mucoperiosteal flaps across the defect and appose with simple interrupted sutures (Figs. 20-18, C and D). Allow the denuded hard palate near the dental arcades to heal by secondary intention. A variation of this technique in which a partial thickness rather than a full-thickness incision of the mucoperiosteum is made without exposure of the palatal bone may reduce maxillofacial deformity. An alternate technique for repair of hard palate defects is the overlapping “sandwich” technique (Figs. 20-19 and 20-20). This technique is advantageous because it does not place the repair over the palate defect. Incise one margin of the defect separating the oral and nasal mucosa (see Fig. 20-20, A). Elevate the mucoperiosteum at this edge approximately 5 mm. At the opposite side of the defect create a mucoperiosteal rotational flap large enough to cover the defect with its base hinged at the margin of the palatal defect (see Fig. 20-20, B). Begin the incision near and parallel to the dental arcade, creating a flap 2 to 4 mm larger than the defect. Make perpendicular incisions at the rostral and caudal end of the incision extending to the cleft. Elevate this mucoperiosteal flap, taking care not to disrupt the margin of the defect (see Fig. 20-20, B). Dissect carefully around the palatine artery to release it from fibrous tissue. Rotate the flap across the defect (see Fig. 20-20, C). Place the edge of the flap under the mucoperiosteal flap on the opposite side. Preplace and then tie a series of horizontal mattress sutures to secure the flaps in position (see Fig. 20-20, D). FIG 20-19 The secondary palate of the puppy in Fig. 20-16 was repaired with an overlapping flap technique. The defect over the hard palate was allowed to heal by second intention. Incise the margins of the cleft to separate the oral and nasal mucosae. Continue incisions made in the margins of hard palate clefts caudally into the soft palate (see Fig. 20-20, D). Isolate the nasal mucosa, palatal muscles, and oral mucosa. Appose the palatal edges in three layers beginning caudally and working rostrally to a point adjacent to the caudal or midpoint of the tonsil. First appose the nasal mucosa using a series of simple interrupted sutures with nasally oriented knots or use a simple continuous pattern. Then appose the palatal muscle and connective tissue with a simple continuous suture pattern. Last, appose the oral mucosa with a simple continuous or interrupted suture pattern. Make tension-relieving incisions in the oral mucosa from the lingual aspect of the last molar to near the tip of the soft palate. Bilateral pharyngeal mucosal flaps may be used to reform the soft palate and have been described in a cat (Headrick and McAnulty, 2004). Create two random-pattern flaps from the right and left pharyngeal walls that are based laterally and extend into the nasopharynx. The base of each flap is at the caudal aspect of the last molar and extends caudally to the rostral margin of the tonsillar crypt. Create a flap of hard palate mucoperiosteum extending from the lateral aspects of the hard palate medial to the dental arcades taking care to preserve the palatine arteries and attachment at the caudal border of the hard palate. Caudal rotation of the mucoperiosteum results in the mucosa facing the new floor of the soft palate. Suture the edges of the flap to the lateral walls of the pharynx. Suture the ends of the lateral pharyngeal flaps together on midline to form the oral mucosal surface of the soft palate. Suture the rostral border of the lateral pharyngeal flaps to the base of the hard palate and the caudal aspect of the three flaps in separate rows of simple continuous patterns. Cosmetic repair of primary clefts can be very complicated, requiring elaborate planning to achieve a successful outcome (Fig. 20-21, A). Create a mucosal flap to separate the nasal cavity from the oral cavity (Fig. 20-21, B). If the cleft extends into the premaxilla, evaluate the gingival mucosa of the deciduous incisors and pull them if necessary. Suture the buccal or gingival mucosal flap to the nasal mucosa. Use a freehand modified Z-plasty for reconstruction of the lip defect (Fig. 20-21, C). Close the lip defect so that the distance from the ventral nostril to the free ventral edge of the lip is the same on both sides. Make multiple small flaps if necessary for a cosmetic closure. Place a layer of sutures in the fibromuscular layer (orbicularis oris muscle and connective tissue) before skin closure. Acquired Oronasal Fistulae Acquired palatal defects are most often caused by dental disease (Fig. 20-22). An oronasal fistula results when a deep maxillary periodontal pocket progresses to the apex of the tooth, lysing bone between the apex of the alveolus and the nasal cavity or maxillary sinus. A fistula may also result from trauma (i.e., bite wounds, gunshot wounds, blunt trauma to the head, electrical burns) or may be a complication of surgery (e.g., mass excision or ventral rhinotomy), radiation, or hyperthermic treatment of oral lesions. Foreign bodies lodged between the dental arcades may cause pressure necrosis of the hard palate and subsequent development of an oronasal fistula (Fig. 20-23). Ingested food that passes through the fistula into the nasal cavity may be expelled from the nostril by sneezing. Chronic rhinitis is common. The diagnosis of an oronasal fistula can be made by identifying an abnormal communication between the oral and nasal cavities (see Figs. 20-22 and 20-23). Small fistulae associated with periodontal disease are not easily identified unless the area around the involved tooth is explored with a narrow dental probe. If passing the probe into the gingival pocket causes epistaxis, a fistula is present. The palatal aspect of the maxillary canine tooth is a common site for an oronasal fistula. Anesthesia generally is required to probe periodontal pockets. Skull radiography or cross-sectional imaging may identify underlying causes of fistulae, such as periapical abscesses, advanced periodontal disease, maxillary neoplasia (see p. 415), or broken or retained tooth roots. Lysis, especially of the laminae dura around tooth roots, is indicative of periapical abscesses. Broad-spectrum antibiotics effective against anaerobes (e.g., chloramphenicol, amoxicillin plus clavulanic acid, clindamycin) should be given if severe purulent rhinitis is present (see Box 20-3). Such animals may benefit from having the nasal infection treated before closure of the defect. With the patient under general anesthesia, cultures should be obtained from the nasal cavity, and the nose and oral cavity flushed of debris and exudate. To prevent recontamination of the nasal cavity with food, the animal should be given nothing by mouth for 10 to 14 days. Nutrition can be provided via tube feeding (e.g., tube gastrostomy, p. 106, or esophagostomy, p. 102) during this time. Most oronasal fistulae require surgical reconstruction, although small or traumatic fistulae occasionally heal spontaneously. A variety of surgical techniques have been described for repair, including simple suturing of the fistula edges, mucosal flaps, mucoperiosteal flaps, double reposition flaps, orbicularis oris axial pattern flaps, palatal island flaps, and two-staged tongue flaps. Successful repair of oronasal fistulae requires a well-supported, airtight, tension-free closure. Flap techniques are more successful than direct apposition of the fistula edges because of less tension and increased support for the repair. Teeth involved in the fistula should be extracted several weeks before reconstruction of the defect. Central lesions may require that normal teeth be extracted to allow creation of adequate mucosal flaps. If the fistula is of dental origin, it may be necessary to perform a limited maxillectomy (at least 5 mm from each margin) to remove necrotic or diseased bone. Traumatic oronasal fistulae may require stabilization of the maxilla and hard palate with small pins or wire. Interdental wiring (see p. 1118) using the carnassial teeth or the canine teeth (or both) can help bring bone edges into apposition. Areas where flaps were harvested heal by second intention in 2 to 3 weeks. Acrylic, Silastic, or metal obturators may also be fitted over the defect. General anesthetic recommendations for animals undergoing oral surgery are provided on page 386 (see Table 20-1 on p. 387). Intubation through a pharyngotomy (see p. 102) or tracheotomy incision (see p. 916) may facilitate repair of large or centrally located oronasal fistulae. Pharyngotomy generally is preferred if it allows adequate visualization of the defect. Care should be taken to prevent and recognize dislodgment of the anesthetic tubing from the endotracheal tube during oral manipulations. Direct apposition of the fistula should be performed only if the fistula is very small. Debride the fistula to healthy, bleeding mucosal edges (Fig. 20-24, A and B). Incise or debride the margin of the fistula and elevate the edges enough to allow approximation without excess tension. Appose the mucosa with interrupted appositional sutures (i.e., simple, cruciate, or vertical mattress pattern) (Fig. 20-24, C).
Surgery of the Digestive System
General Principles and Techniques
Preoperative Concerns
Anesthetic Considerations
![]() TABLE 20-1
TABLE 20-1

 Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the United States.
Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the United States.
Antibiotics
Surgical Anatomy
Surgical Techniques
Temporary Carotid Artery Ligation
Partial Maxillectomy
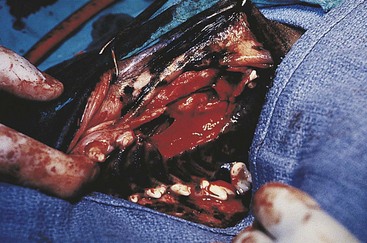
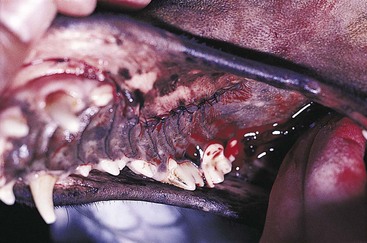
Partial Mandibulectomy
Mandibular Rim Excision
Tonsillectomy
Glossectomy
Pharyngotomy
Salivary Gland Excision
Postoperative Care and Assessment
Complications
Special Age Considerations
References
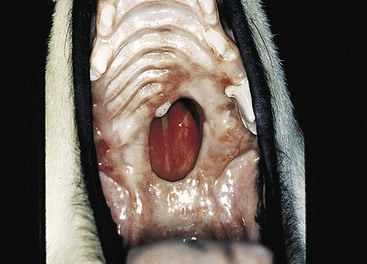
Congenital Oronasal Fistula (Cleft Palate)
General Considerations and Clinically Relevant Pathophysiology
Medical Management
Surgical Treatment
Anesthesia
Surgical Anatomy
Surgical Techniques
![]() Closure of Hard Palate Defects
Closure of Hard Palate Defects
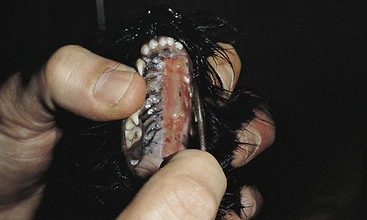
Closure of Soft Palate Defects
Reconstruction of Soft Palate Hypoplasia
![]() Closure of Primary Clefts
Closure of Primary Clefts
General Considerations and Clinically Relevant Pathophysiology
Diagnosis
Physical Examination Findings
Diagnostic Imaging
Medical Management
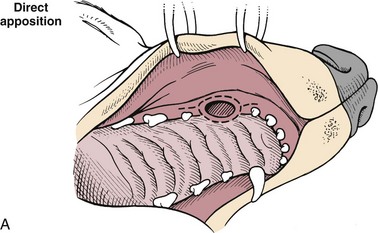
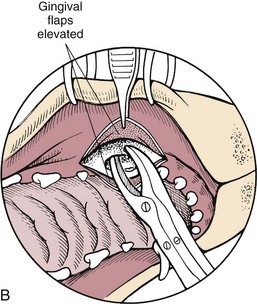
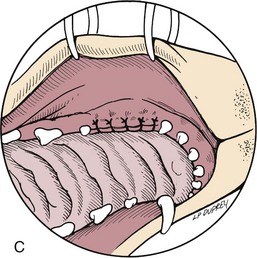
Surgical Treatment
Anesthesia
Surgical Techniques
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Digestive System