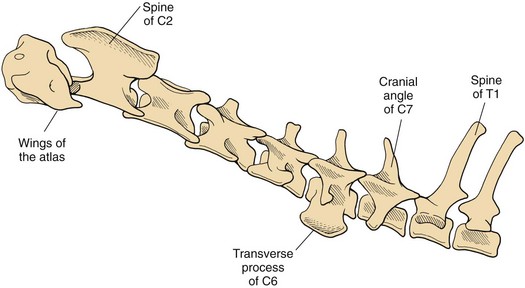
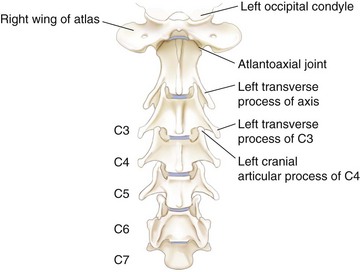
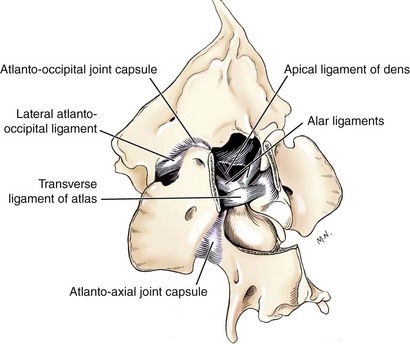
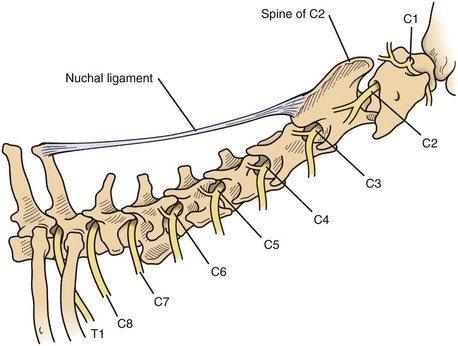
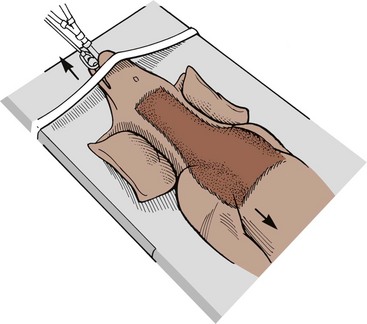
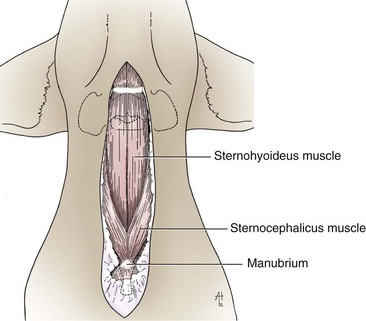
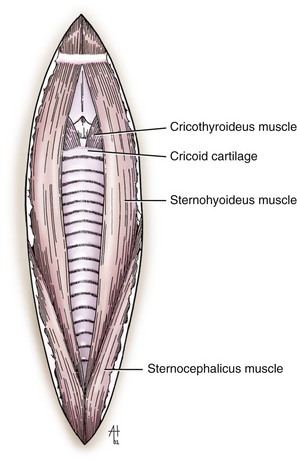
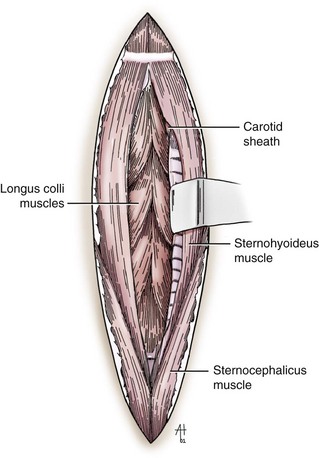
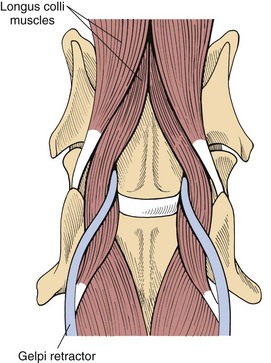
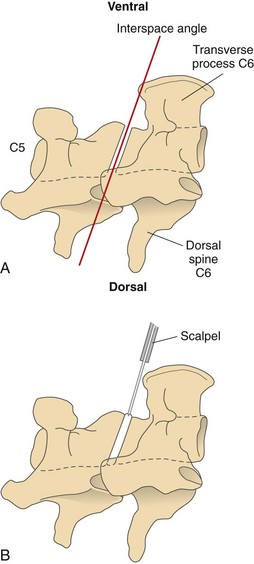
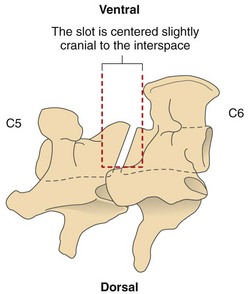
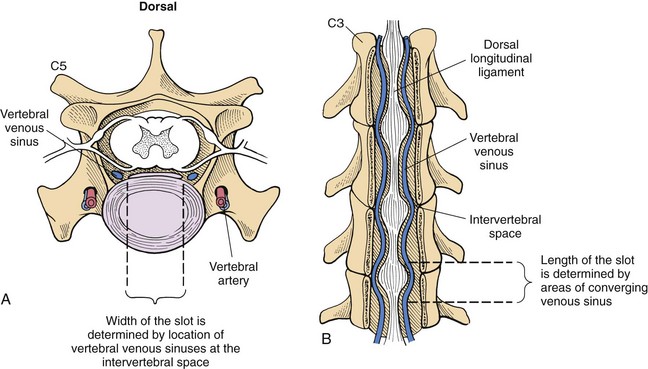
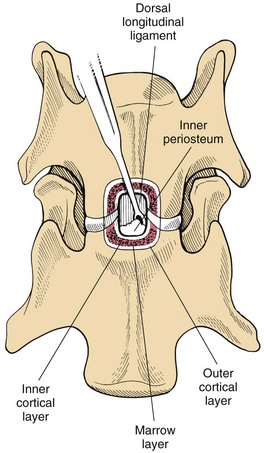
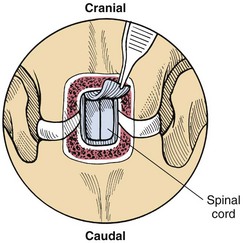
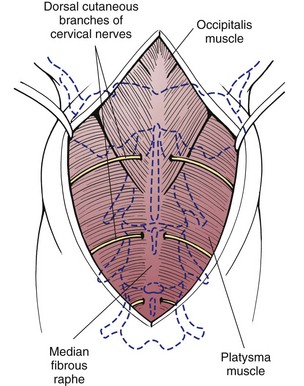
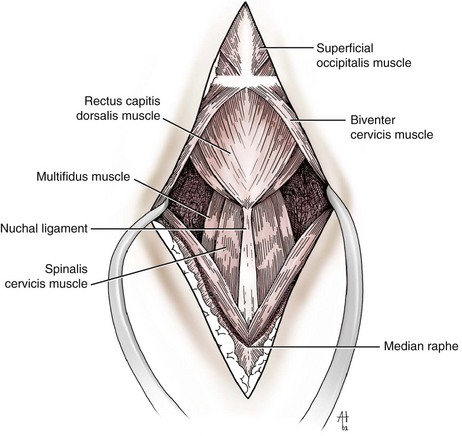
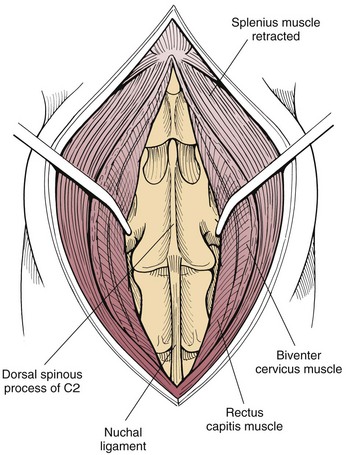
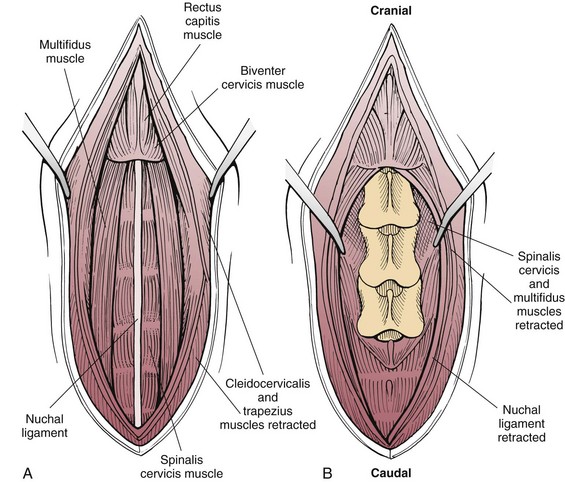
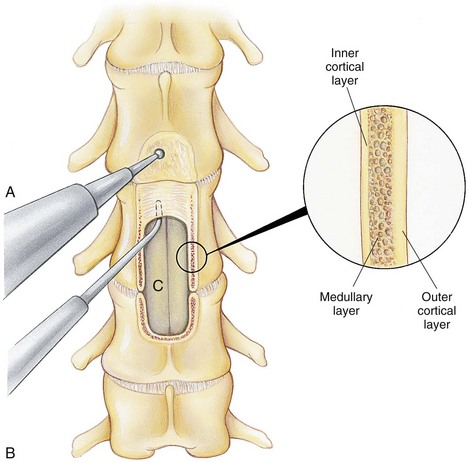
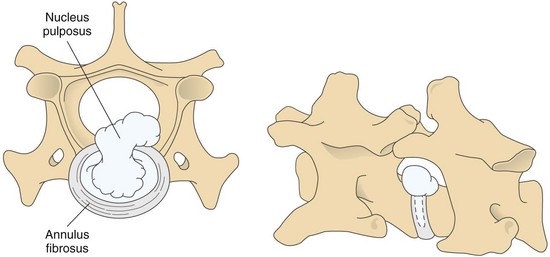
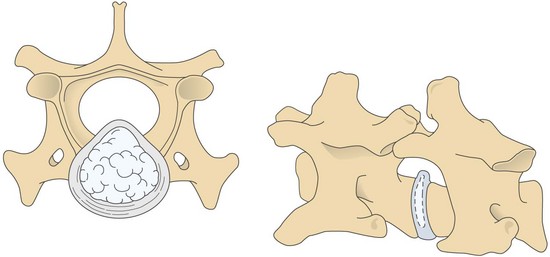
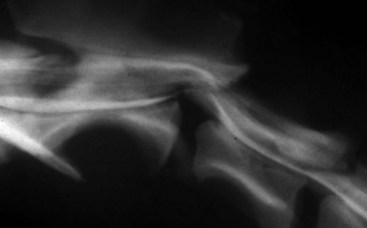
Chapter 40 Weakness of all four limbs due to a cervical myelopathy is tetraparesis. When voluntary motor ability is lost to all four limbs, the term used is tetraplegia. Hemiparesis and hemiplegia are similar terms that refer to weakness of or inability to move the thoracic and pelvic limbs on one side of the body, respectively. The two most common surgical approaches to the cervical spinal cord are the ventral slot procedure (creation of a bony defect in the ventral aspect of an intervertebral space in addition to removing a section of the intervening disk) and dorsal laminectomy. The ventral slot procedure provides a small window to the ventral aspect of the spinal cord, but lateral access is limited by both the size of the defect and the large vertebral venous sinuses (internal vertebral venous plexus) on either side of the defect. Dorsal laminectomy provides a wide decompression and access to the entire dorsal surface of the cord. Further removal of the articular processes laterally (facetectomy) and/or the pedicles allows for exposure of the nerve roots/spinal nerve and lateral surface of the spinal cord, respectively. Removal of parts of the articular processes done to enlarge the intervertebral foramen is called foraminotomy. Removal of the dorsal lamina on one side is often performed, usually combined with facetectomy/foraminotomy; this is called dorsolateral hemilaminectomy. Hemilaminectomy is the unilateral removal of the lateral surface of the vertebral arch, including the articular processes. Although this can be done in the cervical region, the term is used more commonly when discussing surgery of the thoracolumbar spine (see Chapter 41). Fenestration is the creation of a defect (window) in the annulus fibrosus to the level of the nucleus pulposus. The range of neurologic dysfunction possible with cervical myelopathies is broad, in terms of both severity and character. Many patients will present with severe neck pain as the primary or sole clinical sign of dysfunction. Neck pain typically is more severe with cranial cervical spinal cord lesions than with caudal lesions. Loss of motor function does occur with compressive cervical myelopathies but is less common than with thoracolumbar myelopathies, most likely owing to the larger volume of the vertebral canal in the cervical region compared with the thoracolumbar region. Ambulatory patients with caudal cervical myelopathy tend to have obvious pelvic limb weakness and ataxia, with less severe, sometimes even subtle, thoracic limb dysfunction. In many such cases, the thoracic limbs move with short, stilted steps, whereas the pelvic limbs display obvious ataxia. This odd gait is often referred to as a “two-engine gait” because of the disparate nature of the thoracic and pelvic limb movements. Patients with cranial cervical myelopathies typically have more obvious thoracic limb dysfunction. A phenomenon called central cord syndrome is sometimes appreciated in dogs and cats with caudal cervical spinal cord lesions that are confined to the central region of the cord. In this syndrome, the thoracic limbs display severe lower motor neuron (LMN) weakness, but the pelvic limbs are minimally affected or neurologically normal. This occurs because the centrally located lesion interferes with LMNs innervating the thoracic limbs, but it spares the more peripherally located white matter upper motor tracts (UMNs) that innervate the pelvic limb LMNs. Central cord syndrome is most commonly associated with intra-axial lesions like syringomyelia and neoplasia (see p. 1500). A hallmark of many cervical myelopathies is neck pain, which is often severe. Oral prednisone (0.5 mg/kg every 12 hours) is often effective in relieving pain in some cervical myelopathies. In chronic compressive myelopathies, this dose of prednisone will also likely improve clinical signs through its effect on spinal cord edema. High doses of glucocorticoids should not be administered as an emergency or pre-emptive presurgical therapy for spinal cord injury. The “high-dose” methylprednisolone protocol has been ineffective for spinal cord injury and can have deleterious consequences. The hydrophilic polymer polyethylene glycol (PEG; 2 ml/kg of a 30% solution IV over 10 to 20 minutes, repeat dose in 6 hours) may be effective in treating patients with spinal cord trauma. This drug is not commercially available and needs to be prepared by a compounding pharmacy. PEG appears to be devoid of side effects (unlike glucocorticoids), but its efficacy is controversial. In animals with severe neck pain, it is preferable to initiate analgesic therapy as early as possible (see Table 12-3 on p. 141). Injectable opioid drugs (e.g., fentanyl) are usually effective in hospitalized patients. Gabapentin and pregabalin are oral calcium channel blocking drugs used as both antiseizure agents and antinociceptive agents (see p. 1440; Table 39-1). Pregabalin is the “next generation” of gabapentin. Although gabapentin is often an effective pain-relieving oral drug, the author has found pregabalin to be superior to gabapentin. Specific anesthetic concerns associated with cervical spinal cord surgery are hypotension, blood loss, cardiac arrhythmias, ventilatory compromise, and pain management. Because of the potential for venous sinus blood loss, blood should be available for transfusion. Severe hypotension—whether due to blood loss, lack of sympathetic tone, or a combination—can be treated with fluid support and/or positive inotropic drugs (see Table 39-2). Bradycardia may occur with vagal nerve stimulation during the ventral approach and can be treated with anticholinergic drugs (atropine or glycopyrrolate). Mechanical ventilation may be necessary both during and after surgery, depending on the severity of the myelopathy. Anesthetic protocols vary but are similar to those used for fracture repair surgery (see Chapter 32, Tables 32-5 and 32-6 on pp. 1045 and 1047). Premedication with an opioid drug is often performed, as many of the cervical myelopathies are characterized by severe neck pain. If myelography is to be performed before surgery, the potential for postmyelographic seizures exists, especially in larger dogs. Antiseizure drugs are discussed in Chapter 39 (see Table 39-1 on p. 1440). Seven cervical vertebrae are present, each of which has a body, laminae, pedicles, transverse processes, and articular processes. All but the first cervical vertebra (C1) have a dorsal spinous process. From C3 to C7, these processes slope in a cranial direction and increase in size from cranial to caudal. No intervertebral disk is found between the first and second cervical vertebrae. Distinctive features of the first (C1), second (C2), and sixth (C6) cervical vertebrae provide important landmarks for the surgeon; similarly, the large dorsal spinous process of the first thoracic vertebra (T1) is a distinct surgical landmark (Fig. 40-1). The first cervical vertebra (C1), the atlas, has a shortened body (the ventral arch); wide, shelflike transverse processes (wings); and modified articular processes. The ventral tubercle of C1 feels like a distinctive “spike” when palpated during a ventral approach (Fig. 40-2), compared with the smooth “bump” of the ventral aspect of the intervertebral disk spaces. The dorsal spinous process of C2 (the axis) is a distinctive bladelike structure, the caudal aspect of which is very thick and partially overhangs the dorsal aspect of C3. The dorsal spinous process of C3 is extremely small and sometimes is nonapparent, especially in miniature and toy dog breeds. The dorsal lamina of C3 in these small breeds may be very short as well. It is important not to confuse C4 with C3 while performing a dorsal approach in the cranial cervical spine, especially in small dogs and cats. The articulation between C1 and C2 involves a cranial projection of the body of C2 (the dens) that rests on and is attached to (via several ligaments) the dorsal aspect of the C1 ventral arch (Fig. 40-3). The transverse processes of C6 are very prominent ventral structures that are easily palpated during the ventral approach. The dorsal aspect of the first rib as it articulates with T1 can also be palpated during the ventral approach; however, the surgeon must be cautious not to rupture the thin cupula pleura during palpation of this structure. In addition to the venous sinuses on the floor of the vertebral canal, a vertebral artery is present on each side of the cervical spine. This large artery traverses the transverse foramina from C6 through C1 and is located ventral to the level of the articular processes from C2 through C6. The vertebral artery usually is not encountered during standard approaches to the cervical spine; disruption of this vessel during surgery can lead to rapid and extensive hemorrhage. Eight paired cervical spinal nerves are present. The paired first cervical spinal nerves exit from the lateral vertebral foramina of C1. The C2 through C7 spinal nerves exit through the intervertebral foramina cranial to the vertebra of the same number as the specific nerve (i.e., the C2 spinal nerve exits at the C1-C2 foramen). The C8 spinal nerve exits the foramen formed by the C7 and T1 vertebrae. The nuchal ligament extends from the T1 dorsal spinous process to the caudal aspect of the C2 dorsal spinous process (Fig. 40-4). This structure is helpful during the dorsal approach both in verifying anatomic location and as a tool for remaining on midline (the nuchal ligament is a paired structure that can be split on the midline). Shave the skin and aseptically prepare the area from approximately the mid-mandible level cranially to several centimeters past the manubrium caudally. The length of the surgical incision depends on the specific area to be operated. Position the patient in dorsal recumbency with the head and neck in mild extension (Fig. 40-5). Secure the thoracic limbs by pulling them caudally and against the patient’s trunk. Use a V-trough or towels to position the neck area. Place a towel beneath the neck to facilitate extension. Secure the head with tape placed over the upper canine teeth. Assuming that the entire cervical region is to be exposed, make a ventral midline incision from the level of the larynx cranially to the level of the manubrium caudally (Fig. 40-6). Divide the sternocephalicus muscles to the level of the manubrium with Metzenbaum scissors or unipolar cautery if access to the caudal cervical spine is required. Divide the paired sternohyoideus muscles on midline (median raphe) with Metzenbaum scissors (Fig. 40-7). During this division, use bipolar cautery to address branches of the caudal thyroid veins that are in the dissection plane. Digitally separate the deep neck fascia, and visually identify then retract the trachea, esophagus, and left carotid sheath to the left and the right carotid sheath to the right. Once these structures are retracted, visualize the longus colli musculature (Fig. 40-8) and perforate the overlying fascial covering with Metzenbaum scissors; remove this fascia digitally using anatomic reference points discussed previously to identify the intervertebral space of interest. Bluntly separate the longus colli musculature on midline at the cranial and caudal aspects of the disk space (for spaces C2-C3 and caudally), using a straight Kelly or mosquito forceps, while exposing the tendons of insertion of the longus colli muscles on the ventral tubercles of the vertebrae. Dissect these tendon insertions from the tubercles using Freer elevators (small dogs and cats), or cut them with Mayo scissors (large dogs). Continue the dissection of the longus colli musculature off the ventral aspect of the vertebral bodies to be exposed using Freer elevators or narrow Army/Navy osteotomes (large dogs). Once this dissection is complete, place Gelpi retractors in the cranial and caudal aspects of the area of interest, with the tips of the retractors beneath the longus colli musculature (Fig. 40-9). Control hemorrhage during this approach using bipolar cautery. When approaching the C1-C2 intervertebral space, transect the belly of the sternothyroideus muscle to facilitate exposure of this joint space. Using a No. 11 blade, incise the small muscle (rectus capitis ventralis) over the ventral surface of C1 and reflect it laterally with Freer elevators to expose the C1 ventral arch. To perform the ventral slot procedure, first fenestrate the disk of interest by resecting a rectangular section of the ventral annulus, and remove this piece of annulus with Lempert rongeurs, exposing the nucleus pulposus (Fig. 40-10, A and B). Remove the ventral tubercle from the caudal aspect of the cervical vertebra forming the cranial aspect of the intended slot. Center the slot toward the vertebral body cranial to the disk because of the caudal-to-cranial direction of the intervertebral space from ventral to dorsal (Fig. 40-11). Do not exceed one-third the width or length of the vertebral bodies when creating the ventral slot. Using a high-speed air drill with a 4- to 5-mm burr, remove the outer cortical and inner cancellous bone layers, noting the spongy red nature of the cancellous layer during drilling. Stay on midline throughout creation of the slot to minimize the chance of rupturing the venous sinuses when entering the vertebral canal (Fig. 40-12, A and B). If the cancellous bone bleeding is enough to interfere with visualization, use bone wax to stop the hemorrhage. When the bone again turns white, the inner cortical layer has been reached. Use a smaller burr (e.g., 2 to 3 mm) to remove this final bone layer. Be cautious to avoid abruptly breaking through this layer. The inner cortical bone takes on a flaky appearance—much like filo dough—just before the vertebral canal is penetrated. Use a probe (e.g., Gross ear hook and spoon) to get under the dorsal annulus, and incise this last layer of the disk with a No. 11 blade. The adjacent periosteal layer is often incised concurrently if the inner cortical layer is thin enough. Once the inner cortical bone layer is thin enough, or if there is a window into the vertebral canal, use a 4-0 bone curette to enlarge the slot defect. In small breed dogs, a distinct dorsal longitudinal ligament may not be apparent (may be removed with the periosteal layer); in larger dogs, this dull white fibrous structure may be distinct (Fig. 40-13). Carefully incise the dorsal longitudinal ligament with a No. 11 blade, if necessary, to gain entry into the vertebral canal (Fig. 40-14). Fibrous (annular) material at the sides of the slot defect will have a frayed appearance, like irregularly cut rope. Calcified nucleus pulposus will appear white and granular, like miniaturized cottage cheese. The dura mater will be smooth and glistening and may be a bright white color or bluish-white (bruising from disk rupture). Remove disk material within the vertebral canal using a probe and Bishop-Harmon forceps so that the dura is visualized. Use the probe to “sweep” underneath the edges of the slot to remove as much disk material as possible. During disk removal and sweeping around the slot defect, it is common to lacerate a venous sinus. Control venous sinus hemorrhage as previously described. After copious lavage of the surgical site, close the longus colli muscles with simple interrupted sutures. If a caudal exposure was performed, reappose the sternocephalicus muscles with simple interrupted sutures. The sternothyroideus muscle does not need to be reattached if this muscle was severed during C1-C2 exposure. Subcutaneous and skin closure is routine. For all of the dorsal approaches, place the patient in sternal recumbency with the neck gently flexed in a neutral position (Fig. 40-15). When the approach to the cranial cervical spinal cord is combined with suboccipital craniotomy (see p. 1446), flex the neck more acutely. The lateral limits of the shaved and aseptically prepared skin area extend to the middle third to half of the dorsal-ventral neck region on each side. The cranial limit of this area for cranial cervical and midcervical approaches is the external occipital protuberance. For midcervical and caudal cervical approaches, the caudal limit of the surgical site is approximately at the level of the dorsal spinous process of T2 or T3. The cranial limit of the surgical site for caudal cervical approaches is approximately at the level of C3. For a cranial cervical approach, incise the skin on the dorsal midline from the external occipital protuberance to the midcervical region (C4 or C5 dorsal spinous process level). Locate the midline fibrous raphe of the superficial cervical musculature (Fig. 40-16). Incise this connective tissue with a No. 11 blade, and carefully continue the midline incision cranially and caudally with Metzenbaum or Mayo scissors, controlling hemorrhage with monopolar and/or bipolar cautery. Reflect and retract the superficial cervical musculature to expose the paired splenius muscles. Divide these muscle bellies as well as those of the underlying biventer cervicis muscles to expose the next layer of paired muscles—the rectus capitis dorsalis muscle cranially and the spinalis cervicis muscle caudally (Fig. 40-17). Identify the nuchal ligament extending caudally from the caudal aspect of C2 at this level. Using a combination of sharp and blunt dissection, elevate the rectus capitis dorsalis muscle off of the C2 dorsal spine. Sharply transect the attachment of the nuchal ligament off the caudal aspect of C2, and divide the muscle bellies of the spinalis cervicis muscles over C3. Using a Freer elevator, bluntly elevate these muscles laterally, exposing the dorsal lamina of C3 (Fig. 40-18). The approach for access to the C1-C2 cervical region is discussed in Chapter 39 (suboccipital craniotomy) (see p. 1446). The initial dorsal approach to the midcervical and caudal cervical regions is similar to that for the cranial cervical approach, with incision of the median raphe of the superficial cervical muscles and retraction of these muscles laterally. To access the dorsal laminae of C4 through C7, divide the nuchal ligament on midline and retract the paired components laterally with Gelpi retractors, exposing the underlying epaxial musculature. Using Mayo scissors, circumferentially cut the tendinous attachments of the spinalis cervicis and multifidus muscles to the dorsal spinous processes, then bluntly elevate these muscles off the dorsal lamina with Freer elevators (small dogs and cats) or Army/Navy osteotomes (large and giant dog breeds). Remove attachments of the multifidus muscles to the articular processes using a combination of periosteal elevators and bipolar cautery (Fig. 40-19, A and B). Remove the dorsal spinous processes of the desired surgery site with rongeurs or bone cutters. Use a high-speed air drill to create the laminectomy defect. The defect should extend the length of the vertebrae of interest and should extend laterally to the level of the articular processes (Fig. 40-20, A through C). In medium to giant breed dogs, the bone will change in color and character as in the ventral slot procedure (i.e., outer cortical, inner cancellous, inner cortical). In cats and small breed dogs, the dorsal lamina in the cervical region often is very thin, and an obvious inner cancellous bone layer may not be apparent. Neurosurgical instrumentation required for cervical spinal surgery is similar to that discussed for brain surgery (p. 1446). For dorsal laminectomies in the cervical region, deep right-angled Gelpi retractors are helpful in providing exposure. Angled burr guards are also needed to create a shelf under a ventral slot for anchoring polymethylmethacrylate (PMMA) in the PMMA plug technique for caudal cervical spondylomyelopathy (CCSM) (see p. 1486). Critical care observation for the first 24 hours postoperatively includes monitoring respiration, administering analgesics, and observing for gastric dilatation-volvulus (particularly in large and giant breed dogs) and seizure activity (particularly if the patient had preoperative myelography). Blood gas analysis is warranted in animals with ventilatory compromise. Postoperative analgesia is provided by low doses of opioids, thus reducing their respiratory depressant effects (see Table 12-3 on p. 141). Fluids should be administered at maintenance rates and the patient turned every 2 hours until sternal. Corticosteroid administration is generally discontinued postoperatively. If neurologic status deteriorates after surgery, corticosteroids may be given until the cause is determined or corrected. Ambulatory patients may be discharged 3 to 5 days after surgery and should be confined for 2 to 4 weeks. They should walk on a harness for 4 to 8 weeks. Nonambulatory patients are treated with frequent hydrotherapy, physiotherapy, an elevated padded cage rack, frequent turning, and bladder expression three to four times a day. They should be kept clean and dry to prevent decubital ulcers. Avoid indwelling urinary catheters because they are a frequent cause of urinary tract infection. A supporting cart helps nurse patients to an ambulatory status. Advantages of a cart include unhindered eating, drinking, micturition, and defecation. Additionally, animals with motor function are encouraged to ambulate, and an erect position facilitates physiotherapy. Nonambulatory patients may be discharged when owners are able to care for them. Neurologic examinations should be performed at 1, 2, 3, 6, 9, and 12 months postoperatively, or until improvement has ceased. Specific Diseases Degenerative disk disease is a common problem in dogs but a relatively infrequent clinical disorder in cats. Two types of disk degeneration (type I and type II) typically cause two distinct types of disk disease (Figs. 40-21 and 40-22). In chondroid (type I) degeneration, the normally gelatinous nucleus pulposus loses water-binding capacity, undergoes degradation of the glycosaminoglycan components, and often becomes calcified. The dorsal annulus often weakens, and the abnormal nucleus pulposus contents extrude through the weakened annulus into the vertebral canal. The severity of spinal cord damage caused by type I disk extrusion is believed to be related to the rate of extrusion (force of impact or concussion), the duration of compression, and the amount of disk material extruded. Fibroid (type II) degeneration involves progressive thickening of the dorsal annulus fibrosus, which protrudes dorsally into the vertebral canal over an extended period of time. Type I and type II degeneration can occur concurrently; these terms do not impose limitations on the pathologic behavior of abnormal disks. The phenomenon of “acute-on-chronic” disk ruptures occurs occasionally, in which a dog with chronic signs of probable type II protrusion worsens rapidly owing to sudden extrusion of nucleus pulposus into the vertebral canal (type I extrusion). In small breed dogs, type I cervical disk disease usually affects cranial cervical disks (C2-C3 most commonly) and typically causes severe neck pain, usually with inapparent or mild neurologic deficits. In large nonchondrodystrophic dogs, the most common site for type I cervical disk extrusions is at the C6-C7 intervertebral disk space; these dogs also tend to present with acute onset of severe neck pain. In the author’s experience, neck pain tends to be more severe with cranial versus caudal cervical disk extrusions. The patient often adopts a “nose-down” posture with an arched back (Fig. 40-23). When turning, these dogs tend to move the head and the neck as one unit, rather than bending at the neck. Fasciculations of the neck musculature can often be appreciated, especially upon palpation of the neck. Clinical signs of a root signature may be apparent. Type II cervical disk disease may result in clinically appreciable neck pain but rarely to the degree encountered in type I cervical disk disease. Type II cervical disk disease usually causes slowly progressive paresis. This can occur as an isolated process or as a component of caudal cervical spondylomyelopathy. Impaired ambulatory function can range from unilateral thoracic limb lameness to tetraplegia with respiratory compromise. Traditional spinal imaging for pets with suspected disk disease consists of plain radiographs followed by myelography (Fig. 40-24), with both performed under general anesthesia. CT and MRI have been found to be useful imaging modalities in the diagnosis of intervertebral disk disease (Figs. 40-25 and 40-26). In some instances (e.g., lateralized type I extrusions), CT or MRI may be preferred. MRI is rapidly becoming the standard imaging modality for dogs and cats with suspected intervertebral disk disease. In addition to providing superior anatomic detail in disk disease patients compared with myelography and CT, MRI is the best modality for diagnosing other spinal disorders that may have similar clinical presentations as intervertebral disk disease and is associated with fewer side effects than myelography. Normally, the nucleus pulposus has high signal intensity on T2-weighted images (the annulus is hypointense). The nucleus pulposus of a degenerative disk is hypointense, and the distinction between the nucleus and the annulus fibrosus may be lost. Results of complete blood count (CBC) and serum biochemical profile analyses are typically normal. A stress leukogram may be observed on CBC results as a result of underlying disease, prior administration of corticosteroids, or a combination of these factors. Hepatic enzyme elevations may be evident with dogs that have been treated with glucocorticoids. Urinary tract infection may be present, especially in dogs treated with dexamethasone. Pyuria and hematuria may or may not be present if the patient is still receiving steroids. Even if collected before myelography is performed, cerebrospinal fluid (CSF) is often not evaluated if clear evidence of a disk compression is present on imaging. Results of CSF analysis with type I disk extrusions are variable. Although CSF findings in type I disk extrusions have classically been characterized as normal to mildly inflammatory, moderate to marked pleocytosis with increased protein concentration is a common finding (Windsor et al, 2008). Administering anti-inflammatory drugs to a patient who is exhibiting signs of an extruded disk without concurrently confining that patient is contraindicated. Anti-inflammatory drugs alleviate the patient’s pain, and most dogs will subsequently become more active. Increased activity is thought to cause more pressure to be placed on the abnormal disk by adjacent vertebrae; subsequently, more disk material is extruded into the vertebral canal, and clinical signs acutely worsen. It is also contraindicated to concurrently administer steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs) to disk disease patients because this combination increases the chances of severe gastrointestinal complications. It is important to realize that subclinical gastroduodenal ulceration is likely to be present in dogs with type I disk extrusions, even without administration of potentially ulcerogenic drugs (e.g., NSAIDs, glucocorticoids); therefore, the use of such drugs should be minimized (see also p. 490). Traditional recommendations for medical management of dogs with type I disk extrusions have recently been called into question, especially those dealing with cage confinement and glucocorticoid administration. In a retrospective report evaluating medical management of dogs with presumptive type I cervical disk extrusions, no association was found between duration of cage confinement and success of medical therapy (Levine et al, 2007). Additionally, no beneficial effect of glucocorticoid administration on the success of medical management was reported. Use of NSAIDs was positively associated with successful outcome. These findings suggest that an extended cage confinement recommendation often is not adhered to by dog owners, and that it may be an excessive recommendation in the first place. The average confinement period was approximately 2 weeks in this study, which may be a more realistic recommendation. In addition to anti-inflammatory medications, oral opiates may be considered for dogs experiencing discomfort (e.g., tramadol, 2 to 4 mg/kg every 8 to 12 hours) and/or pregabalin (2 mg/kg PO every 12 hours).
Surgery of the Cervical Spine
General Principles and Techniques
General Considerations
Preoperative Management
Anesthetic Considerations
Surgical Anatomy
Standard Surgical Approaches to the Cervical Spine
Ventral Approach to the Cervical Spine
Dorsal Approach to the Cervical Spine
Suture Materials and Special Instruments
Postoperative Care and Assessment
Cervical Disk Disease
General Considerations and Clinically Relevant Pathophysiology
Diagnosis
Physical Examination Findings
Diagnostic Imaging
Laboratory Findings
Medical Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Cervical Spine