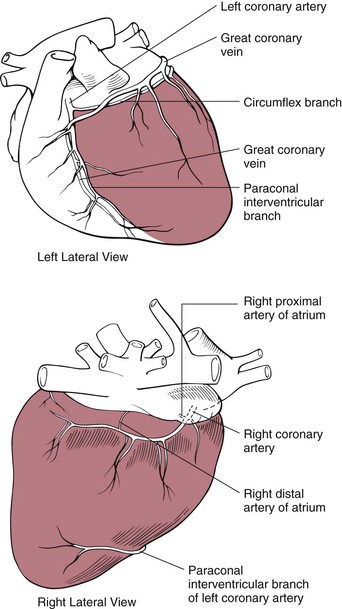
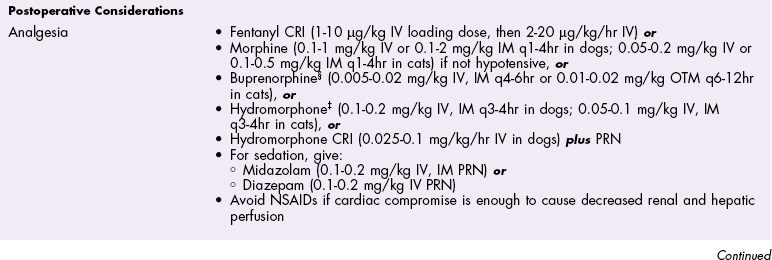
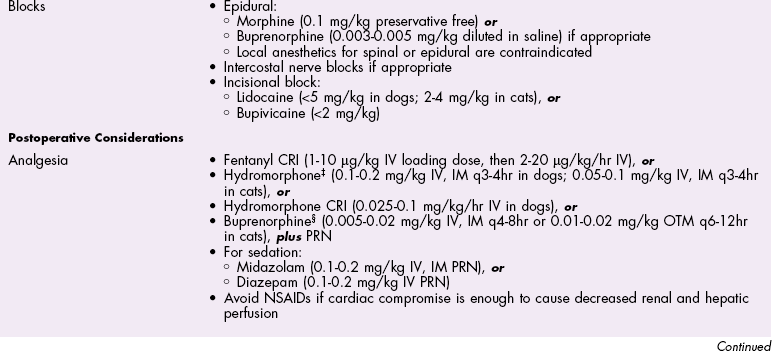
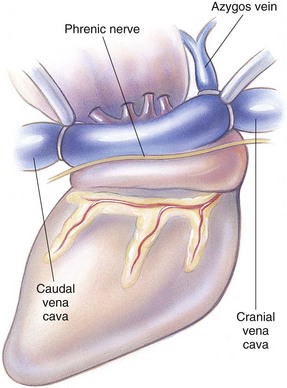
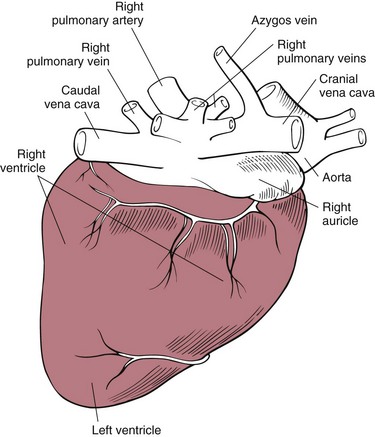
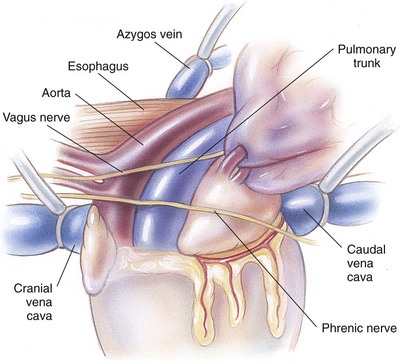
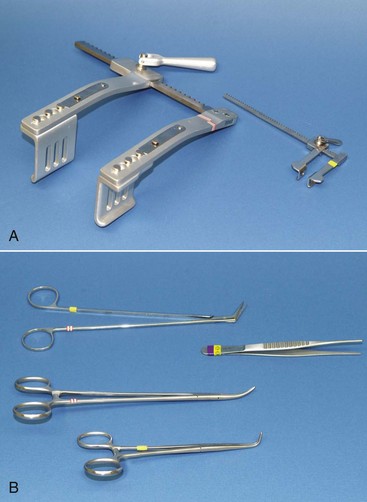
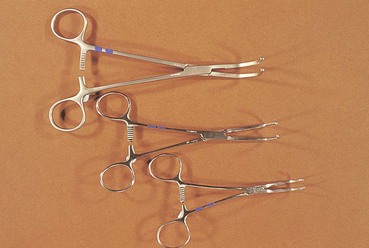
Chapter 28 General Principles and Techniques Animals requiring cardiac surgery often have prior cardiovascular compromise that should be corrected or controlled medically when possible before anesthetic induction (Box 28-1). Congestive heart failure (CHF), particularly pulmonary edema, should be managed with diuretics (e.g., furosemide) and angiotensin-converting enzyme (ACE) inhibitors (e.g., enalapril, benazepril, lisinopril) and an inodilator (pimobendan) before surgery. Cardiac arrhythmias should be recognized and treated (see also later discussion in the “Postoperative Care and Assessment” section). Ventricular tachycardia should be suppressed before surgery with class I antiarrhythmic drugs (i.e., lidocaine and procainamide). Other antiarrhythmic drugs to consider include sotalol and amiodarone. Lidocaine is effective for management of ventricular tachyarrhythmias during and immediately after surgery. Supraventricular tachycardia may require digoxin, β-adrenergic blockers (e.g., esmolol, propranolol, atenolol), or calcium channel–blocking drugs (e.g., diltiazem) before surgery. Atrial fibrillation should be controlled before surgery with a β-blocker or a calcium channel blocker with or without digoxin to lower the ventricular response rate to below 140 beats per minute. Alternatively, amiodarone may be used to control the ventricular response rate, and in a small percentage of cases, to convert atrial fibrillation to normal sinus rhythm. Animals with bradycardia should undergo an atropine or glycopyrrolate response test before surgery. If bradycardia is not responsive to atropine or glycopyrrolate, temporary transvenous pacing or constant intravenous infusion of isoproterenol (see management of bradycardia on p. 901) may be required. Anesthesia of the patient with cardiac compromise has risks that vary depending on the cause of the underlying disease. For example, the anesthetic protocol that is safest for the patient with mitral regurgitation can be dangerous for the patient with aortic stenosis. The pathophysiology of the patient’s cardiac condition needs to be fully understood. Likewise, the practitioner needs to have a working knowledge of the pharmacology of the drugs used to manipulate heart rate and blood pressure. Although cardiac surgery is typically performed at veterinary teaching hospitals and referral institutions, veterinarians from a variety of practices are required to anesthetize the patient with heart disease. See p. 870 for a discussion of anesthesia in patients with mitral regurgitation and p. 881 for information on anesthesia in the patient with subaortic stenosis (Tables 28-1 to 28-3). See also the discussion of anesthesia for the patient with cardiac tamponade on p. 892. Cardiovascular Parameters for Patients with MR, SAS, or Tamponade Adapted from Jaffe RA, Samuels SI: Anesthesiologist’s manual of surgical procedures, ed 4, Philadelphia, 2009, Lippincott Williams & Wilkins. Anesthetic Considerations in the Patient with Mitral Regurgitation* *Patients with compromised cardiac output have longer drug onset times. Be patient and wait for adequate circulation time before repeating a dose. †Should be considered in invasive abdominal/thoracic surgeries and other long procedures. ‡Monitor for hyperthermia in cats. Anesthetic Considerations in the Patient with Aortic Stenosis or Subaortic Stenosis* *Patients with compromised cardiac output have longer drug onset times. Be patient and wait for adequate circulation time before repeating a dose. †Should be considered in invasive abdominal/thoracic surgeries and other long procedures. ‡Monitor for hyperthermia in cats. Induction of anesthesia should be undertaken with caution in animals with cardiopulmonary compromise. Thiobarbiturates should be avoided in patients with significant cardiac disease because they result in dose-dependent cardiac depression and are arrhythmogenic. Propofol (Diprivan or Rapinovet) produces rapid induction but causes essentially the same cardiovascular compromise as thiobarbiturates. The addition of fentanyl decreases propofol requirements in healthy dogs with minimal alteration in cardiovascular parameters (Covey-Crump and Murison, 2008). Ketamine combined with diazepam may be appropriate for induction of compromised patients. It should be avoided in animals with mitral insufficiency because it increases the regurgitant fraction by increasing peripheral vascular resistance. However, it is the induction agent of choice in animals with pericardial constriction. Opioids can be used for induction of very sick and compromised dogs; however, opioids do not induce hypnosis, so intubation may be difficult. Etomidate is not arrhythmogenic, maintains cardiac output, and offers rapid induction, although it is associated with longer and poorer recoveries (Sams et al, 2008). Mask induction is discouraged in all patients with cardiopulmonary disorders because the patient’s reduced cardiac output will cause an increased time to achieve adequate induction. Additionally, inhalants cause marked hypotension, which is often undesirable. A balanced anesthetic approach using benzodiazepine, opioids, and modest amounts of inhalant is generally much safer. Atracurium (Box 28-2) is a short-acting muscle relaxant that is not dependent on metabolism or excretion to terminate its action; it may be used if further muscle relaxation is needed (it must be used with intermittent positive-pressure ventilation [IPPV]). Successful inflow occlusion requires meticulous anesthesia. Intraoperative and postoperative care may be complicated and may require multiple vasoactive medications. For further discussion of anesthesia techniques for these patients, the reader is referred to a cardiac anesthesia text. Balanced anesthetic techniques that minimize inhalation anesthetic agents are indicated (e.g., fentanyl citrate plus atracurium besylate combined with isoflurane [see Box 28-2]). Administration of a single dose of dexamethasone (see Box 28-2) after induction may be beneficial in reducing cardiac damage and improving postoperative outcomes (Yared et al, 2007). Mild hypothermia (32° C to 34° C) reduces basal metabolic rate, allowing lengthening of occlusion time; however moderate hypothermia (<32° C) is associated with spontaneous ventricular fibrillation. Animals should be hyperventilated for 5 minutes before inflow occlusion. Ventilation is discontinued during inflow occlusion and is resumed immediately upon reestablishment of blood flow. Drugs and equipment for full cardiac resuscitation must be available immediately after inflow occlusion. Gentle cardiac massage may be necessary after inflow occlusion to reestablish cardiac function. Digital occlusion of the descending aorta during this period helps direct available cardiac output to the heart and brain. If ventricular fibrillation occurs, immediate internal defibrillation is necessary as soon as inflow occlusion is discontinued. Constant intravenous infusion of lidocaine (see Box 28-2) should be initiated before inflow occlusion and continued as necessary. Epinephrine, administered as a constant rate infusion, should be given as the animal is being weaned off inflow occlusion or a pump (see Box 28-2). If long-term inotropic support is necessary, dobutamine or amrinone should be given (see Boxes 28-1 and 28-2 and Tables 28-1 through 28-3). Perioperative antibiotics are indicated for cardiac procedures lasting longer than 90 minutes. First-generation cephalosporins (e.g., cefazolin, cephapirin) can be administered intravenously at induction and repeated once or twice (Box 28-3). For cardiac procedures involving circulatory arrest or cardiopulmonary bypass, intravenous cefoxitin should be administered before surgery and continued for 24 hours after surgery owing to impairment of humoral host defenses associated with these procedures (see Box 28-3). The right atrium receives blood from the systemic circulation. The coronary sinus enters the left caudal aspect of the atrium, ventral to the caudal vena cava. The caudal vena cava returns blood from the abdominal viscera, the pelvic limbs, and a portion of the abdominal wall (Fig. 28-1). The cranial vena cava returns blood to the heart from the head, neck, thoracic limbs, and ventral thoracic wall, and from a portion of the abdominal wall. The azygos vein usually enters into the cranial vena cava; it carries blood from the lumbar regions and the caudal thoracic wall. The brachycephalic trunk is the first large artery from the aortic arch. The common carotid arteries usually arise from it as separate vessels. The left subclavian artery arises from the aortic arch distal to the brachycephalic trunk (the right subclavian is a branch of the brachycephalic trunk). The vertebral arteries, costocervical trunk, internal thoracic arteries, and axillary arteries branch from the subclavian vessels. Cardiac surgery is not fundamentally different from other types of general surgery, and similar principles of good surgical technique (i.e., atraumatic tissue handling, good hemostasis, and secure knot tying) apply. Consequences of poor surgical technique are often devastating. Cardiac surgery differs from other surgeries in that motion from ventilation and cardiac contractions adds to the technical difficulty of performing these procedures. Approaches that provide limited access to dorsal structures (e.g., median sternotomy; see p. 967) require that surgeons incise, suture, and/or ligate structures located deep within the thorax. Ligature placement using hand ties (see p. 76) is useful in such situations, and the ability to place hand-tied knots (vs. instrument tying) should be considered a fundamental skill for cardiac surgeons. Secure knot tying is critically important for successful cardiac surgery. Hand tying of knots is fast and produces tighter and more secure knots than instrument tying. The one-handed knot tie technique (see p. 78) is best suited to the fine sutures used in cardiac surgery. Tight knots are facilitated by throwing the first two or three throws in the same direction before finishing with square knots for security. Closure of cardiovascular structures requires precise suturing techniques and good instrument handling skills to minimize hemorrhage. Using fine suture with swaged-on atraumatic needles (see discussion on suture materials on p. 865) and carefully following the needle contour when suturing (to minimize the size of needle tracts) are important. “Palming” of needle holders is a good skill for fast suturing but should be avoided when suturing inside the thoracic cavity. Finer control is gained by grasping instruments with fingers placed in the instrument rings. Depending on the cardiac procedure that is being done, perform a left or a right thoracotomy (see p. 964) or a median sternotomy (see p. 967). With a right thoracotomy or median sternotomy, occlude the cranial and caudal vena cava and the azygos vein with vascular clamps or Rumel tourniquets (Fig. 28-2). Make a Rumel tourniquet by passing umbilical tape around the vessel, then thread the umbilical tape through a piece of rubber tubing that is 1 to 3 inches long. When the umbilical tape has been adequately tightened to occlude the vessel, place a clamp above the rubber tubing to hold it securely in place. Take care to prevent injuring the right phrenic nerve during placement of the clamps or tourniquets. For left thoracotomies, pass separate tourniquets around the cranial and caudal venae cavae. Then, while dissecting dorsal to the esophagus and aorta, occlude the azygos vein by placing a tourniquet around it (Fig. 28-3). FIG 28-2 To occlude cardiac inflow from the right side of the thorax, pass tapes around the caudal vena cava and the common drainage of the azygos veins and the cranial vena cava. Fashion tourniquets as described in Figure 28-3. Successful cardiac surgery requires proper surgical instrumentation. Most of the basic instruments required for general surgery can be used for cardiac surgery; however, a few specialized instruments are desirable for thoracic surgery. The standard thoracic retractor is a Finochietto retractor (Fig. 28-4, A). It is helpful to have retractors of at least two sizes to accommodate animals of different sizes. Self-retaining orthopedic retractors can substitute as thoracic retractors in small dogs and cats. The standard tissue forceps for thoracic surgery is a DeBakey tissue forceps (Fig. 28-4, B). At least two DeBakey forceps should be available, and it is helpful if one has a carbide inlay for grasping suture needles. Metzenbaum scissors are the standard operating scissors for cardiac surgery. Curved Metzenbaum scissors are more versatile than the straight design. Potts scissors (45-degree angle) are desirable for some cardiac surgery procedures (see Fig. 28-4, B). Needle holders should be long and available in different sizes to accommodate a variety of suture needle sizes. Mayo-Hegar, Crile-Wood, and Castroviejo needle holders represent a good selection of sizes for thoracic surgery in animals. Angled thoracic forceps are an important instrument for cardiac surgery and should be available in a variety of sizes (see Fig. 28-4, B). Vascular clamps are noncrushing clamps used for temporary occlusion of cardiovascular and pulmonary structures. They come in a variety of sizes and shapes, including straight, angled, curved, and tangential (Fig. 28-5). The most versatile shape for most cardiac surgery procedures is a medium-width tangential clamp. Evaluation of ventilation is important after any thoracic surgery. Poor ventilatory efforts may first be noted in the period after surgery, when the influence of anesthetic drugs is still present but ventilatory support has been discontinued. Hypoventilation may also result from uncontrolled pain. Total ventilation can be assessed directly by measuring the volume of expired gas with a respirometer. Tidal volume should be at least 10 ml per kg of body weight. Ultimately, the best measure of alveolar ventilation is arterial CO2 tension (PaCO2). Alveolar hypoventilation is present when PaCO2 is increased to above 40 mmHg. Treatment of hypoventilation should be directed at correcting its underlying cause if possible. Drugs that are known to depress ventilation (i.e., opioids and muscle relaxants) should be used with caution in the perioperative period, and the risk of ventilatory depression weighed against the risk of hypoventilation due to pain (see p. 961 for analgesia after thoracotomy). Pleural air or fluid should be evacuated if present. Injury or dysfunction of the neuromuscular ventilatory apparatus should be corrected, if possible. If hypoventilation is severe and the cause is not immediately correctable, positive-pressure ventilation is indicated. If necessary, keep the animal intubated and ventilated postoperatively until partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), pH, blood pressure (BP) and heart rate (HR) indicate that the patient is stable enough to extubate. Constant-rate infusions of fentanyl and propofol can assist in keeping the patient comfortable until complications such as significant ventilation/perfusion ( Systemic blood pressure is directly proportional to cardiac output and systemic vascular resistance. Measurement of blood pressure provides a good assessment of cardiovascular function, especially during and immediately after surgery. Indirect techniques for measuring blood pressure include the oscillometric method, which serves as the basis of monitors such as the Dinamap, or Doppler, method. Doppler technique provides only systolic pressure but is useful for evaluating blood pressure trends during and after surgery. Indirect methods of blood pressure assessment are less invasive, but are also less accurate than direct measurements. Direct measurement of blood pressure requires placement of an arterial catheter. Arterial catheters have the additional advantage of providing access for arterial blood gas analysis. An arterial catheter can be placed percutaneously into a dorsal pedal artery. Direct blood pressure measurement also requires a pressure transducer and a monitor, or a manometer. The therapeutic goal is to maintain a mean blood pressure above 65 mmHg and systolic blood pressure above 90 mmHg. Blood pressure can be elevated by increasing cardiac output or systemic vascular resistance. In many instances (depending on the cause), a more appropriate therapeutic strategy to correct hypotension is to improve cardiac output. Maintenance of adequate vascular volume is the most important aspect of maintaining adequate cardiac output. Central venous pressure should be maintained at between 5 and 10 cm of water. If echocardiography is available, evaluation of ventricular filling is a more reliable way to detect hypovolemia. Indications for arterial pressor therapy are rare. Inotropic and pressor support can be obtained by constant intravenous infusion of epinephrine (see Box 28-2). Long-term inotropic support is maintained with dobutamine (see Box 28-2). Monitoring the electrocardiogram for disturbances in cardiac rhythm is important for animals undergoing cardiac surgery. Sinus tachycardia is the most common rhythm disturbance in surgery patients. Therapy for sinus tachycardia should be directed at correction of its underlying cause (e.g., hypovolemia, pain, anxiety, acidosis, hypotension, anemia, hypoxemia, drug-induced) and improvement in cardiac output. Ventricular dysrhythmias, including premature ventricular complexes (PVCs) and nonsustained or sustained ventricular tachycardia, are frequently encountered during and after cardiac surgery. Frequent PVCs, particularly when they occur with a short coupling interval (i.e., R-on-T phenomena), and rapid ventricular tachycardia should be suppressed in the perioperative period. Continuous intravenous infusion of lidocaine is effective in most instances. Ventricular fibrillation is a form of cardiac arrest that requires immediate electrical defibrillation. If cardiac surgery is performed, equipment for defibrillation should be available. Recommendations for postoperative analgesics are provided in Chapter 12 (see Table 12-3 on p. 141 and Box 31-2 on p. 992). The major complication associated with cardiac surgery is hemorrhage. Severe hemorrhage may be encountered intraoperatively or postoperatively. Materials for blood transfusion should be available (see Box 4-1 and Table 4-5 on pp. 30 and 34, respectively). Fresh whole blood should be collected as close as possible to the time that it is needed and should not be cooled because this may reduce platelet content. If possible, a compatible donor should be identified by cross-matching the patient before surgery. Cell Saver autologous blood recovery systems (Haemonetics, Braintree, Mass.) are available for collection and processing of blood for procedures in which rapid bleeding or high-volume blood loss may occur. They can also be used to sequester platelets and plasma from a patient immediately before surgery, thereby reducing the need for donor blood. Covey-Crump, GL, Murison, PJ. Fentanyl or midazolam for co-induction of anaesthesia with propofol in dogs. Vet Anaesth Analg. 2008;35:463. Sams, L, Braun, C, Allman, D, et al. A comparison of the effects of propofol and etomidate on the induction of anesthesia and on cardiopulmonary parameters in dogs. Vet Anaesth Analg. 2008;35:488. Yared, JP, Bakri, MH, Erzurum, SC, et al. Effect of dexamethasone on atrial fibrillation after cardiac surgery: prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2007;21:68. Specific Diseases The clinical presentation and progression of MVD are variable. Most dogs remain asymptomatic for their lifetime. For dogs that do have progression of disease, volume overload of the left ventricle occurs when blood regurgitates through the mitral valve, causing left atrial and left ventricular hypertrophy. As the mitral valve annulus dilates, MR typically becomes more severe, and left-sided CHF typically ensues. Atrial fibrillation may be associated with left atrial dilation, especially in large and giant breeds of dogs with sufficient atrial mass to sustain the arrhythmia. A classification system has been proposed (Box 28-4) to aid identification of dogs with cardiac disease, and to link the extent of clinical signs with appropriate treatment and monitoring recommendations. Affected animals typically have a holosystolic murmur heard best at the left cardiac apex. The intensity of the murmur may be correlated with the severity of disease in some dogs (usually small breeds) (Ljungvall et al, 2009). Pulmonary crackles may be heard if pulmonary edema is present. Electrocardiographic evidence of left atrial and/or left ventricular enlargement may be manifested by a P wave duration greater than 0.04 second (P mitrale) or tall R waves (>2 to 2.5 mV), respectively, in lead II. Few data suggest that medical intervention before the onset of clinical signs of heart failure is beneficial, and the topic remains controversial (Atkins et al, 2009). In fact, several placebo-controlled trials have demonstrated that therapy with ACE inhibitors or inodilators has no effect on the symptom-free interval (time to onset of heart failure) in asymptomatic patients, although benazepril was shown to have a beneficial effect in certain breeds in an uncontrolled retrospective study (Atkins et al, 2007; Quellet et al, 2009; Pouchelon et al, 2008). Once CHF is documented, treatment with diuretics (e.g., furosemide; see Box 28-1), ACE inhibitors (i.e., enalapril or benazepril; see Box 28-1), and inodilators (pimobendan, see Box 28-1) is indicated. Additional diuretics (e.g., spironolactone, hydrochlorothiazide) and vasodilators (e.g., hydralazine, amlodipine) should be considered in refractory cases. The use of β-blockers in dogs with MVD is controversial because they can exacerbate unstable CHF; a recent study found quality of life and functional class improvement in a small number of dogs, although echocardiographic variables were unchanged (Marcondes-Santos et al, 2007). Further investigation is warranted. Preoperative arrhythmias should be controlled before surgery. Affected animals typically have signs of CHF, and treatment with positive inotropes (i.e., digoxin or pimobendan), vasodilators (i.e., hydralazine, enalapril, or amlodipine), and diuretics (i.e., furosemide or spironolactone) is indicated (see previously in “Medical Management” section). The goal of managing the patient with mitral regurgitation while the patient is under anesthesia is to maximize forward flow of blood and minimize the regurgitant fraction (see Tables 28-1 and 28-2). To do this, heart rate needs to be kept at high normal values and blood pressure needs to be normal to 20% below normal. A slower heart rate allows for larger filling volumes, potentially leading to left ventricular distention and dilation of the mitral annulus. This can result in a larger regurgitant fraction. Treat bradycardia with anticholinergics (i.e., atropine, glycopyrrolate). Patients with mild or moderate disease usually have adequate left ventricular function and normal end-diastolic ventricular pressures, especially in mild to moderate disease. Therefore they are able to perfuse the myocardium with low-normal systemic blood pressures. With lowering of systemic vascular resistance (SVR), the pressure gradient across the aortic valve favors forward flow of blood into the aorta. This reduces the regurgitant fraction, increases cardiac output, and increases blood pressure. High systemic blood pressure should be avoided. If it does occur, hypertension should be treated immediately by deepening the anesthesia with an inhalational agent and giving a rapidly acting opioid (i.e., fentanyl). If the initial treatment is inadequate, nitroglycerin or nitroprusside may be titrated to effect as a constant rate infusion. To avoid increases in pulmonary vascular resistance (PVR) and worsening pulmonary hypertension, avoid acidosis, nitrous oxide, hypoxemia, hypoventilation, and hypercarbia. Refer to Tables 28-1 and 28-2 for anesthetic recommendations for patients with mitral regurgitation undergoing cardiac or noncardiac procedures. The right and left coronary vessels (Fig. 28-6) arise from the aortic bulb immediately distal to the aortic valve. The right coronary artery arises from the right sinus of the aorta and curves to the right and ventrocranially, lying in the fat of the coronary groove. Its initial part is bounded by the pulmonary trunk and the conus arteriosus craniolaterally; dorsally it is covered by the right auricle. The left coronary artery is a short trunk about 5 mm long and nearly as wide. It terminates in the circumflex and paraconal interventricular branches. The circumflex branch lies in the coronary groove as it extends to the left. On approaching the dorsal interventricular groove, it turns toward the apex of the heart and is known as the subsinuosal interventricular branch. The combined length of the circumflex and subsinuosal branches is approximately 8 cm in the dog. The paraconal interventricular branch is approximately 1.5 mm wide and 7 cm long. It winds obliquely and distally from left to right across the sternocostal surface of the heart in the paraconal interventricular groove. Postoperative pain should be treated with systemic opioids and local anesthetic techniques (see Box 31-2 on p. 992 for post-thoracotomy analgesia). Animals should be monitored for pulmonary edema after surgery. If pulmonary edema occurs, it should be treated with furosemide (see Box 28-1). Left ventricular failure should be treated as outlined in the “Medical Management” section. MVD is a common cardiac condition, but most dogs remain asymptomatic during their lifetime. For dogs with progressive disease and heart failure, prognosis is related to cardiac size and severity of MR, type of pharmacologic and adjunctive therapy, cardiac cachexia, complications, and other concomitant disease (Borgarelli et al, 2008). Mitral valve replacement with a mechanical valve prosthesis was shown to have a median survival after surgery of 4.5 months (Orton et al, 2005). Although most dogs survived the surgery, a high incidence of prosthetic valve thrombosis was reported. Experimental studies with the current generation of bioprosthetic valves show improved antithrombogenicity, but further investigation is warranted (Takashima et al, 2008). Mitral valve repair (e.g., circumferential annuloplasty, placement of artificial chordae, chordal fenestration, papillary muscle splitting, edge-to-edge repair) successfully resolved signs of CHF in 75% of dogs that survived surgery, for a median period of 1 year (range, 4 months to 3 years) after surgery (Griffiths et al, 2004). Atkins, C, Bonagura, J, Ettinger, S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142. Atkins, CE, Keene, BW, Brown, WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc. 2007;231:1061. Borgarelli, M, Savarino, P, Crosara, S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120. Griffiths, LG, Orton, EC, Boon, JA. Evaluation of techniques and outcomes of mitral valve repair in dogs. J Am Vet Med Assoc. 2004;15:224. Ljungvall, I, Ahlstrom, C, Höglund, K, et al. Use of signal analysis of heart sounds and murmurs to assess severity of mitral valve regurgitation attributable to myxomatous mitral valve disease in dogs. Am J Vet Res. 2009;70:604. Marcondes-Santos, M, Tarasoutchi, F, Mansur, AP, Strunz, CM. Effects of carvedilol treatment in dogs with chronic mitral valvular disease. J Vet Intern Med. 2007;21:996. Orton, EC, Hackett, TB, Mama, K, et al. Technique and outcome of mitral valve replacement in dogs. J Am Vet Med Assoc. 2005;226:1508. Ouellet, M, Bélanger, MC, Difruscia, R, et al. Effect of pimobendan on echocardiographic values in dogs with asymptomatic mitral valve disease. J Vet Intern Med. 2009;23:258. Pouchelon, JL, Jamet, N, Gouni, V, et al. Effect of benazepril on survival and cardiac events in dogs with asymptomatic mitral valve disease: a retrospective study of 141 cases. J Vet Intern Med. 2008;22:905. Takashima, K, Soda, A, Tanaka, R, et al. Long-term clinical evaluation of mitral valve replacement with porcine bioprosthetic valves in dogs. J Vet Med Sci. 2008;70:279. Patent Ductus Arteriosus The characteristic physical examination findings (i.e., continuous murmur and bounding arterial pulses) make diagnosis of PDA straightforward in most affected animals. Rarely, a combination of aortic stenosis and/or aortic insufficiency (see p. 879), or VSD and/or aortic insufficiency (see p. 882), causes a to-and-fro murmur that may be difficult to differentiate from continuous PDA murmurs. In animals in which the diastolic component of the PDA murmur is difficult to detect, other differentials would include subaortic stenosis, pulmonic stenosis (PS), atrial septal defect (ASD), and VSD. Differentials for dogs with right-to-left PDA include tetralogy of Fallot, right-to-left shunting, ASD or VSD, and other rare complex forms of cyanotic heart disease. Animals with pulmonary edema should be given furosemide (see Box 28-1) for 24 to 48 hours before surgery. If atrial fibrillation is present, the ventricular response rate should be controlled with a β-adrenergic blocker or a calcium channel blocker (with or without digoxin) or amiodarone before surgery. If hemodynamically significant arrhythmias are present, they must also be controlled. Complete resolution of clinical signs of CHF may be difficult or impossible with medical management alone. Long-term medical management of dogs with right-to-left PDA has been described in only a small number of dogs using phlebotomy or hydroxyurea. Intravascular coils, vascular plugs, and duct occluders are now used routinely for closure of patent ductus arteriosus (Fig. 28-7). These techniques have the advantage of not requiring a thoracotomy and have less risk for major complications; however, mortality rates are comparable between transcatheter arterial occlusion and surgical ligation. Transversus coil embolization has been reported in dogs weighing fewer than 3 kg (Henrich et al, 2011). Ductal occlusion is most commonly performed from access through the femoral artery, although recently coil embolization through the carotid artery has been described, as has a transvenous approach through the femoral vein (Blossom et al, 2010; Miller and Thomas, 2009). The coil(s) or occluders are placed in the ductus under fluoroscopic guidance, and complete occlusion is verified by injection of contrast agent into the aorta (Fig. 28-8).
Surgery of the Cardiovascular System
Preoperative Management
Anesthesia
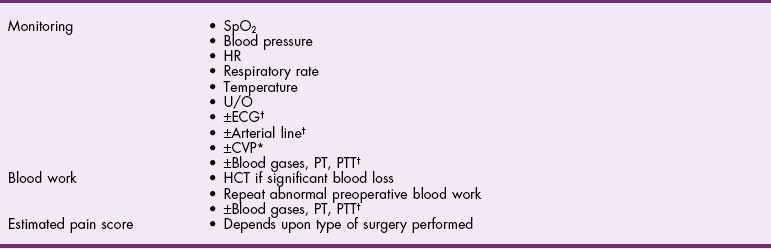
![]() TABLE 28-1
TABLE 28-1

![]() TABLE 28-2
TABLE 28-2


![]() TABLE 28-3
TABLE 28-3


Antibiotics
Surgical Anatomy
Surgical Technique
Inflow Occlusion
Suture Materials and Special Instruments
Postoperative Care and Assessment
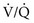 ) mismatch or acidosis have improved. If necessary, vasopressors may be administered to correct hypotension caused by propofol.
) mismatch or acidosis have improved. If necessary, vasopressors may be administered to correct hypotension caused by propofol.
Complications
References
Mitral Regurgitation
General Considerations and Clinically Relevant Pathophysiology
Diagnosis
Physical Examination Findings
Differential Diagnosis
Medical Management
Surgical Treatment
Preoperative Management
Anesthesia
Surgical Anatomy
Postoperative Care and Assessment
Prognosis
References
Differential Diagnosis
Medical Management
Surgical Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Cardiovascular System

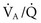 ) mismatch with formation of pulmonary shunts secondary to alveolar collapse. The importance of
) mismatch with formation of pulmonary shunts secondary to alveolar collapse. The importance of 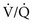 ratios relates to how well the lungs resaturate venous blood with O2 and eliminate CO2. During procedures involving circulatory arrest or cardiopulmonary bypass, ventilation is temporarily ceased and the remaining oxygen is absorbed, resulting in collapsed alveoli (absorption atelectasis). With reperfusion of collapsed pulmonary tissues, blood passes through the lungs without becoming oxygenated. The result can be large shunts of deoxygenated blood returning to the left side of the heart. Patients with shunts are not responsive to higher concentrations of oxygen. Instead, they need assisted ventilation and positive end-expiratory pressure (PEEP). Therefore, response to supplemental oxygen therapy must be evaluated for each individual patient, preferably by arterial blood gas analysis. The therapeutic goal of supplemental oxygen should be to keep PaO2 above 80 mmHg. Ventilation and PEEP are indicated for patients with severe gas exchange impairment that is not responsive to supplemental oxygen therapy alone.
ratios relates to how well the lungs resaturate venous blood with O2 and eliminate CO2. During procedures involving circulatory arrest or cardiopulmonary bypass, ventilation is temporarily ceased and the remaining oxygen is absorbed, resulting in collapsed alveoli (absorption atelectasis). With reperfusion of collapsed pulmonary tissues, blood passes through the lungs without becoming oxygenated. The result can be large shunts of deoxygenated blood returning to the left side of the heart. Patients with shunts are not responsive to higher concentrations of oxygen. Instead, they need assisted ventilation and positive end-expiratory pressure (PEEP). Therefore, response to supplemental oxygen therapy must be evaluated for each individual patient, preferably by arterial blood gas analysis. The therapeutic goal of supplemental oxygen should be to keep PaO2 above 80 mmHg. Ventilation and PEEP are indicated for patients with severe gas exchange impairment that is not responsive to supplemental oxygen therapy alone.