15 In 1978 Yoxall1 stated: “It is surprising, for instance, how much a dog’s quality of life, observed by the owner, may be improved by the administration of a simple analgesic if the dog is suffering from a tumor, which although painless on palpation, may be causing considerable chronic pain.” Despite this statement and the fact that obvious pain associated with specific tumors such as osteosarcoma (OSA) has been emphasized for a long time as a diagnostic criterion, there is little literature specifically investigating cancer pain in companion animals.2–14 However, it is very encouraging that since the previous edition of this book, there has been an approximately fourfold increase in published studies (from 4 to 17 using a PubMed search). Encouragingly, we have seen the first studies looking at mechanisms in companion animal cancer pain.15,16 This chapter will deal with the treatment of chronic cancer pain in dogs and cats. Given the relative lack of clinical work in dogs and cats, the information in this chapter cannot be based on peer-reviewed investigations. Rather, it is a combination of the authors’ experience and the experience of others who are heavily involved in the treatment of cancer patients. It is also based on considered extrapolations from human medicine and from veterinary research in other chronically painful conditions, such as osteoarthritis. The control of acute perioperative pain in cancer patients is also very important (see section on relationship between cancer and pain), and readers are referred to appropriate texts for information on perioperative pain control.17 Not all tumors are painful, and the amount of pain is likely to vary considerably from one animal to another, even with similar tumor types. The author’s experience and the experience of others would suggest that, using a conservative estimate, 30% of tumors in dogs and cats are associated with significant pain at the time of diagnosis. Tumors most likely to be associated with pain include those at the following sites: oral cavity, bone, urogenital tract, eyes, nose, nerve roots, gastrointestinal tract, and skin (Table 15-1). The 30% estimate is likely conservative since pain is experienced by 20% to 50% of human patients when the lesion is diagnosed, by nearly half undergoing active treatment, and by up to 90% with far advanced or terminal cancer. An overall average of about 70% of human patients with advanced cancer suffer pain.18 Table 15-1 List of Tumors Most Likely to Be Associated with Pain The incidence of pain in animals caused by cancer is very difficult to estimate, as is the effectiveness of analgesic therapy. Recent surveys have found that significant numbers of animals in the perioperative setting were not receiving analgesic drugs,19–26 although an overview of these studies seems to suggest the situation is improving. Analgesics are even less likely to be used for cancer pain. Glucocorticoids do provide some analgesia, and their use may be more widespread,22 but the specific treatment of cancer pain in animals is still likely to be suboptimal. • Lack of appreciation that many cancers are associated with significant pain. • Overly focusing on the cancer treatment, rather than patient comfort. • Inability to assess pain in cancer patients. • Lack of knowledge of drugs, drug therapy, and other pain-relieving techniques • Lack of communication with clients and lack of involvement of clients in the assessment and treatment phases. • Under-use of nursing staff for assessment and reevaluation of pain in cancer patients. Although drug treatment is the mainstay of cancer pain treatment, effective cancer pain treatment often involves a combination of drug therapy, nondrug therapies, and good communication between all parties involved. A basic approach to cancer pain management is summarized in Table 15-2. Table 15-2 A Basic Approach to Cancer Pain Management The alleviation of pain is important not only from a physiologic and biologic standpoint, but also from an ethical point of view.27 To help in an evaluation of welfare, “five freedoms,” initially proposed by the Brambell Committee in reference to farmed animals,28 have been suggested. They may be applied equally in the context of companion animals27 and are as follows: • Freedom from hunger and thirst • Freedom from physical and thermal discomfort • Freedom from pain, injury, and disease • Freedom to express normal behavior Pain relief may affect survival in cancer patients. Immunosuppression has been shown to depend on the severity of surgery in clinical29–31 and experimental animal studies.32,33 Additionally, the severity of surgery has been shown to influence tumor metastasis.34–37 In 2002, a human clinical study suggested that laparoscopic colectomy was associated with increased survival,38 and although subsequent studies found no difference in long-term survival,39 there is interest in the immunologic and oncologic implications of less-invasive surgery.40 In 2001, Page and coworkers found that the provision of analgesics significantly reduced the tumor-promoting effects of undergoing and recovering from surgery in a rodent model.41 The reduction of the tumor-promoting effects of surgery by analgesics may result from maintenance of natural killer (NK) cell function, but it is likely that other unrecognized factors also play a role.41,42 More recently, it was found that spinal analgesia attenuated the laparotomy-induced suppression of tumoricidal function of liver mononuclear cells and decreased metastasis compared to rodents undergoing laparotomy without spinal analgesia.43 This was considered to be due to preservation of T helper 1/T helper 2 (Th1/Th2) cytokine balance. Thus the provision of adequate perioperative pain management in oncologic surgery may be protective against metastatic sequelae in clinical patients. It is quite possible that chronic pain experienced by animals with cancer may also affect metastasis. Assessment of pain in animals, while often difficult, is extremely important. It is likely that the tolerance of pain by an individual animal varies greatly and is further complicated by the innate ability of dogs and cats to mask significant disease and pain. Physiologic variables such as heart rate, respiratory rate, temperature, and pupil size are not reliable measures of acute perioperative pain in dogs44 and are unlikely to be useful in chronic pain states. The mainstay of pain assessment in cats and dogs suffering from cancer is likely to be changes in behavior. Table 15-3 outlines behaviors that are probably indicative in certain situations of pain. In general, if a tumor is considered to be painful in humans, it is appropriate to give an animal with a similar condition the benefit of the doubt and treat it for pain. Table 15-3 The most important people in the assessment process are the owners. The veterinarian must work closely with the owner to capture this information. Often, owners need to be educated as to what signs to look for and that certain behaviors may be indicative of pain. Once very specific changes in behavior can be identified and recorded, these behaviors can be used to monitor the effectiveness of analgesic therapy. Furthermore, these specific activities can be used to create goals and therapy tailored to try to meet these goals. The importance of patient-reported outcomes (PROs) in human medicine is widely recognized.45 These PROs may refer to a large variety of different health data reported by patients, such as symptoms, functional status, quality of life (QOL), and health-related quality of life (HRQOL).45 In humans, QOL is a complex, abstract, multidimensional concept defining an individual’s satisfaction with life in domains he or she considers important. The term HRQOL reflects an attempt to restrict this complex concept to those aspects of life specifically related to the individual’s health and potentially modifiable by healthcare.46 Both QOL and HRQOL have been used in veterinary medicine. Questionnaires have been developed to assess HRQOL in dogs47,48 and cats49 with cancer. Although pain certainly appears to be assessed in these HRQOL tools, it is not known how specific or sensitive these tools are to changing pain status. • Nonopioid analgesics (e.g., nonsteroidal antiinflammatory drugs [NSAIDs], acetaminophen) • Weak opioid drugs (e.g., codeine) • Strong opioid drugs (e.g., morphine) • Adjuvant drugs (e.g., corticosteroids, tricyclic antidepressants, anticonvulsants, N-methyl d-aspartate [NMDA] antagonists). A general outline to approaching cancer pain is given in Figure 15-1. This figure can be used in combination with Tables 15-4 and 15-5. There are many different scenarios that the clinician may face when dealing with cancer pain, and this figure and these tables are only guides. The term wind-down therapy refers to admitting refractory pain patients into the hospital and treating them with multiple intravenous (IV) analgesic medications in the hope that the pain can be controlled, making it easier to subsequently manage the pain at home. Table 15-4 Suggested Doses of Analgesics that May Be Used for the Alleviation of Chronic Cancer Pain in the Dog PO, By mouth; GI, gastrointestinal; NSAIDs, nonsteroidal antiinflammatories; IV, intravenous. None of these drugs have been evaluated for efficacy in the treatment of cancer pain. Table 15-5 Suggested Doses of Analgesics that May Be Used for the Alleviation of Chronic Cancer Pain in the Cat None of the drugs have been evaluated for efficacy in the treatment of cancer pain. The drugs that can be used for chronic cancer pain management are outlined in Tables 15-4 and 15-5. The following notes are not a comprehensive appraisal of each class of drug but are suggestions for their use for cancer pain. NSAIDs have been the mainstay of therapy for chronic pain, especially in osteoarthritis, and they are an excellent first line of treatment in cancer pain. There are several excellent reviews on NSAID use in small animals, and the reader is referred to these.50–54 The choice of NSAIDs available can be bewildering, but a few key points are as follows: • On a population basis, all NSAIDs are probably equally efficacious in relieving pain, but for a given patient, one drug is often more effective than another. • Gastrointestinal side effects associated with NSAID use appear to be more common with drugs that preferentially block COX-1 over COX-2. • There is no difference in renal toxicity between COX-1 selective drugs and COX-2 selective drugs. • Liver toxicity can occur with any NSAID. • There are no completely safe NSAIDs, but the approved NSAIDs are significantly safer than the older nonapproved NSAIDs. • Longer term or continuous NSAID use appears to be more effective than short-term or reactive use,52 but in relatively stable disease states, gradual dose reduction may be possible while maintaining efficacy.55 There are no licensed NSAIDs for long-term administration in cats other than meloxicam, which is approved in the European Union for long-term treatment of musculoskeletal pain. However, a number of these compounds can probably be used safely (see Table 15-5). The key to safe chronic NSAID administration in cats is the use of the smallest effective dose and avoiding use or using decreased doses in cats with renal disease. Choosing drugs with short half-lives is also considered important by the author. Acetaminophen is a nonacidic NSAID; many authorities do not consider it an NSAID as it probably acts by somewhat different mechanisms.56 With any chronic pain, there are always CNS changes, so for what seems a “peripheral” problem such as many cancers, centrally acting analgesics can be very effective. Although highly toxic in the cat, even in small quantities, it can be effectively used in dogs for pain control in the acute setting.57,58 No studies of toxicity in dogs have been done, but if toxicity is seen, it will probably affect the liver; thus the drug, in common with all NSAIDs and opioids, should be used cautiously in dogs with liver dysfunction. It can be used on its own or in a preparation combined with codeine and is initially dosed at about 10 to 15 mg/kg twice a day (bid). The author often uses it as the first-line analgesic therapy in dogs with renal compromise in which NSAIDs cannot be used or in dogs that appear to be otherwise intolerant to NSAIDs (e.g., vomiting or gastrointestinal ulceration). Opioids can be an effective part of the management of cancer pain, particularly when used as part of a multimodal approach (i.e., including NSAIDs or adjunctive analgesics). Side effects of opioids include diarrhea, vomiting, sedation, and constipation with long-term use. It is often constipation and occasionally the sedation that owners object to most as a side effect in their pet. Oral morphine, transdermal fentanyl, oral butorphanol, sublingual buprenorphine (cats only), and oral codeine are used most often for the alleviation of chronic cancer pain. None of these drugs has been fully evaluated for clinical toxicity when administered long term nor for efficacy against chronic cancer pain. Dosing must be done on an individual basis, and adjustment of the dose to produce effective analgesia without undesirable side effects requires interaction and communication with clients. However, despite these suggestions, recent evidence has indicated that oral opioids may not reach effective plasma concentrations in dogs when dosed at the currently recommended levels.59–62 There is currently no information on the long-term use of oral opioids for chronic pain in the cat. There appears to be individual variation in the level of analgesia obtained with certain opioids, especially morphine and butorphanol, in the acute setting.63,64 Buprenorphine appears to produce predictable analgesia when given sublingually65 and is well accepted by most cats. The small volume required (max 0.066 mL/kg [20 µg/kg]) makes administration simple. Based on clinical feedback from owners, this is an acceptable technique for home use. Inappetence can occur after several days of treatment and sometimes lower doses (5 to 10 µg/kg) can overcome this problem. When administered concurrently with other drugs, less frequent dosing is often required.65 The NMDA receptor appears to be central to the induction and maintenance of central sensitization,66–68 and the use of NMDA receptor antagonists would appear to offer benefit where central sensitization has become established (i.e., especially chronic pain). Ketamine, tiletamine, dextromethorphan, and amantadine possess NMDA antagonist properties, among other actions. Ketamine is not obviously useful for the management of chronic pain due to the formulation available and the tendency for dysphoric side effects even at low doses. However, oral ketamine has not been evaluated in dogs or cats for long-term administration. Intraoperative microdose IV ketamine appears to provide beneficial effects for a variety of oncology surgical procedures, including limb amputations,69 and this may decrease the incidence of chronic pain later. Other reports suggest a benefit of using ketamine perioperatively in low doses.70 When used in this manner, ketamine should be administered as a bolus (0.5 mg/kg IV) followed by an infusion (10 µg/kg/min) prior to and during surgical stimulation. A lower infusion rate (2 µg/kg/min) may be beneficial for the first 24 hours postoperatively and an even lower rate (1 µg/kg/min) for the next 24 hours. In the absence of an infusion pump, ketamine can be mixed in a bag of crystalloid solutions for administration during anesthesia. Using anesthesia fluid administration rates of 10 mL/kg/hr, 60 mg (0.6 mL) of ketamine should be added to a 1-liter bag of crystalloid fluids to deliver ketamine at 10 µg/kg/min. The active metabolite of dextromethorphan may not be produced in dogs, probably negating its use in the species for chronic pain.61 Amantadine has been used for the treatment of neuropathic pain in humans,71 and one report suggests a benefit of adding amantadine to an NSAID in dogs that do not get complete relief from the NSAID alone.72 Suggested dosages are given in Tables 15-4 and 15-5. The toxic side effects have been evaluated in the dog (but not the cat), and the dosages suggested are considered safe.73 Amantadine should be avoided in patients with congestive heart failure, history of seizure, or those on selegiline, sertraline, or tricyclic antidepressants. Tramadol is a synthetic derivative of codeine and is classified as an opioidergic/monoaminergic drug.74,75 It has been found to be effective in the alleviation of pain associated with osteoarthritis in humans. Tramadol’s analgesic efficacy is a result of complex interactions between opiate, adrenergic, and serotonin receptor systems. Hepatic demethylation of tramadol produces the active metabolite, O-desmethyltramadol (M1). The different metabolites interact with different receptors; thus efficacy in different species is likely to depend on the metabolic characteristics in the particular species. Initial work in dogs suggested that tramadol was absorbed sufficiently, producing levels that would theoretically provide analgesia.76 However, recently there has been some discussion about the actual amounts of the M1 metabolite that are formed in dogs, and the pharmacokinetics of tramadol does not favor an analgesic effect.77–81 There is no published evidence of efficacy for canine pain. Little is known about the side effects of tramadol in dogs, and almost nothing is known about the side effects seen when tramadol is combined with other drugs in human or canine medicine. In human medicine, a recent study found that for patients hospitalized for peptic ulcer treatment, tramadol use prior to admission was associated with just as high a risk of mortality as was NSAID use prior to admission. Additionally, mortality was 2.02- and 1.41-fold higher in these groups of patients, respectively, than in patients who used neither tramadol nor NSAIDs.82 A recent study evaluating the analgesic effects of various doses of rofecoxib and tramadol alone and in combination found that the most analgesic combination of tramadol and rofecoxib produced gastric injury in rats that was more severe than with rofecoxib or tramadol alone.83 Tramadol is metabolized differently in the cat84,85 and appears to have clinical efficacy when administered intravenously perioperatively86 and has shown antinociceptive activity when administered orally in an experimental setting.87 The dosages given in Tables 15-4 and 15-5 are for the regular (not prolonged release) form of the drug. It has not been thoroughly evaluated for toxicity in the dog or cat. Many anticonvulsants such as carbamazepine, phenytoin, baclofen, and more recently gabapentin have been used to treat chronic pain, including neuropathic pain in humans, as well as chemotherapy-induced peripheral neuropathies. Gabapentin and the more recently introduced pregabalin appear to be among the most effective drugs available for neuropathic pain in humans. While the exact mechanism of action of these drugs is unclear, one potential mode by which they exert their analgesic effect is by binding to the α2-δ protein subunit of voltage-gated calcium channels, thereby reducing excitatory neurotransmitter release through channel modulation or channel trafficking. Although there is considerable information on gabapentin disposition in dogs88,89 and some information on its use as an anticonvulsant in dogs,90 there is as yet no information on its use for the control of chronic or long-term pain. However, a recent small study suggested no beneficial effect of perioperative gabapentin.91 Information on the kinetics of gabapentin in cats has become available,92 and one study found a lack of thermal antinociception.93 There are no scientific publications demonstrating its efficacy for long-term pain in this species. While the indications for gabapentin (and pregabalin) are presently unclear in veterinary patients, they do appear to be useful for cancer pain in some patients and are probably particularly effective in cancers that have some neurogenic or nerve destruction component. Suggested dosages are in Tables 15-4 and 15-5. Tricyclic antidepressants have been used for many years for the treatment of chronic pain syndromes in people and are becoming widely used for the modulation of behavioral disorders in animals. Within the CNS, there are descending inhibitory serotonergic and noradrenergic pathways that reduce pain transmission in the spinal cord. Tricyclic antidepressants such as amitriptyline, clomipramine, fluoxetine, imipramine, maprotiline, and paroxetine primarily inhibit the reuptake of various monoamines (serotonin for clomipramine, fluoxetine, and paroxetine; noradrenaline for imipramine, amitriptyline, and maprotiline). Tricyclic antidepressants can also interact directly with 5-hydroxytryptamine (5-HT) and peripheral noradrenergic receptors and may also contribute other actions such as voltage-gated sodium channel blockade and reduction in peripheral prostaglandin E2 (PGE2)-like activity or tumor necrosis factor (TNF) production. However, in human medicine, there is a relative lack of controlled, clinical trials specifically evaluating the efficacy of antidepressants in treating cancer pain,94 with the exception of two studies demonstrating a lack of efficacy in the treatment of chemotherapy-induced peripheral neuropathy.95,96 If there are beneficial effects, it could be from modulation of pain or improvement in mood or feeling. The tricyclic antidepressant amitriptyline appears to be effective in the cat for pain alleviation in interstitial cystitis,97 and many practitioners are reporting efficacy in other chronically painful conditions in the cat, including osteoarthritis. Amitriptyline has been used daily for periods up to 1 year for interstitial cystitis, and few side effects are reported. The author has also used amitriptyline in the cat for cancer pain with some encouraging results. It should probably not be used concurrently with other drugs that modify the serotonergic system such as amantadine or tramadol until more is known about drug interactions. Alterations in the level of expression, cellular localization, and distribution of sodium channels are seen in many pain states. These aberrantly expressed sodium channels result in hyperexcitability and ectopic activity in peripheral and central nerves that encode nociceptive information. Low doses of lidocaine and other sodium-channel blockers readily block these aberrantly expressed sodium channels, producing pain relief. Low-dose IV lidocaine has proven as effective as other commonly used medications for treatment of neuropathic pain in humans,98 and the author uses such an approach to “downregulate” central sensitization in veterinary cancer patients. There is increasing interest in the use of transdermal lidocaine patches for treatment of cancer pain.99 Much of this interest revolves around using the patch to administer a low systemic level of lidocaine that blocks the aberrantly expressed sodium channels. Studies have been performed evaluating the kinetics of lidocaine absorbed from patches applied to dogs and cats.100–102 Peak plasma concentrations of lidocaine were obtained between 10 and 24 hours postapplication in dogs and at 65 hours postapplication in cats. The results of these studies indicate that, similar to humans, systemic absorption of lidocaine from the patch is minimal. Peak plasma concentrations were over 100 times below the level reported to induce neurologic signs and 10 times below the level reported to result in myocardial depression in dogs and 25 times lower than that observed following an IV injection of lidocaine (2 mg/kg) in cats. Potential systemic toxicity associated with lidocaine administration, including bradycardia, hypotension, cardiac arrest, muscle or facial twitching, tremors, seizures, nausea, and vomiting, were not noted in any study. Dosing guidelines have been suggested,103 although to date there have been no published reports evaluating the analgesic efficacy of lidocaine patches in veterinary cancer patients, but the technique holds promise. Malignant bone disease creates a unique pain state, with a neurobiologic signature distinct from that of inflammatory and neuropathic pain.104–106 Bone cancer–related pain is thought to be initiated and perpetuated by dysregulated osteoclast activity and activation of nociceptors by prostaglandins, cytokines, and H+ ions. Therapies that block osteoclast activity not only have the potential to markedly reduce bone pain but may also mitigate other skeletal complications associated with neoplastic conditions, including pathologic fractures and hypercalcemia of malignancy. Bisphosphonates are synthetic analogs of pyrophosphate whose primary effect is to inhibit osteoclast activity. Bisphosphonates accumulate in bone, and following osteoclast-mediated bone resorption, bisphosphonates are released and disrupt cellular functions, resulting in osteoclast death. Oral absorption of bisphosphonates tends to be poor, and IV dosing is the preferred route of administration. Adverse effects include nephrotoxicity, electrolyte abnormalities, and acute-phase reactions.107,108 In addition to the inhibitory effects of bisphosphonates on osteoclasts, reports suggest that they may also exert direct effects on cancer cells, including canine OSA and fibrosarcoma lines.109,110 Studies have found beneficial effects of IV pamidronate for the treatment of malignant osteolysis associated with primary and secondary bone neoplasms.10,11,108,111 These studies have also suggested analgesic effects; however, the positive assessments have been based on subjective evaluations. In a placebo-controlled study, there was no difference in the pain relief between placebo and bisphosphonates using either subjective or objective (force plate) evaluations.9 Despite these results, it is believed that some patients attain pain relief from bisphosphonate administration, and a better and validated means of assessing cancer pain may help elucidate the degree of pain relief that can be achieved. Pamidronate may be administered at a dose of 1 to 2 mg/kg as a 2 to 4 hour infusion (diluted in saline) and repeated at 3 to 5 week intervals. Other examples of a bisphosphonate that can be used in dogs are clodronate, alendronate, and zoledronate.111–113 Several new approaches to pain treatment revolve around the use of mechanisms to destroy or exhaust neurons involved in pain transmission. One approach is to use targeted neurotoxins to cause neuronal death.114 An example of this is the combination of a neurotoxin (saponin) to substance P. Substance P, when administered, binds to the neurokinin receptor (NKR), and the conjugate is internalized (a normal phenomenon of the receptor-ligand interaction), resulting in cell death due to the neurotoxin.115,116 Because sensory neurons are rich in NKRs, if the conjugate is targeted appropriately (e.g., given intrathecally), sensory neurons are killed. Basic science studies suggest that in models of chronic pain, general sensory function is left intact, whereas hyperalgesia associated with chronic pain is decreased. Some toxicity work has been performed in dogs,117 and clinical trials in dogs with naturally occurring bone OSA are underway, with these being used as a model of human OSA pain. Another approach uses the transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) to target neurons involved in pain. When drugs such as capsaicin and resiniferatoxin bind to TRPV1, the resulting calcium influx that is initiated results in an inability of the neuron to function. If the activation of TRPV1 occurs for long enough or is intense enough, the resulting calcium influx can cause the neurons to degenerate and undergo apoptosis. Capsaicin is used in humans for neuropathic pain, and resiniferatoxin has been evaluated in dogs with naturally occurring bone cancer pain in which it provided prolonged pain relief, despite significant hemodynamic effects.5 Radiation therapy is considered to palliate pain in canine OSA, although the evidence is largely anecdotal. A 0-7-21 palliative regimen, using 8 or 10 Gy fractions, has been reported to result in pain relief lasting about 70 days.4,118 Additionally, two 8 Gy fractions over 2 consecutive days was reported to result in pain relief for a duration of 67 days.13 The difficulty in interpreting the value of radiation therapy for pain relief in these studies is the lack of controls or objective measures. Indeed, the natural course of pain and lameness in dogs with appendicular OSA has not been determined. One study evaluating objective measure of limb use in dogs with OSA undergoing radiation therapy found no significant changes in kinetic parameters after one 8-Gy dose of radiation.2 Samarium is a radioisotope that has been evaluated for use in dogs.119 Although the use of samarium Sm153 lexidronam in veterinary medicine is still limited, results of a noncontrolled clinical study with subjective assessments suggested improvement in lameness scores in 63% of dogs, suggesting that this therapy may be useful in the palliation of pain in dogs with bone tumors in which curative-intent treatment is not pursued.3 Acupuncture can be provided through simple needle placement or by needle placement combined with electrical stimulation (of high or low frequency, although most types of pain respond to low-frequency stimulation). Results of a study in normal experimental dogs demonstrated a weak analgesic effect of electroacupuncture in anesthetized patients as evaluated by a reduction in the minimum alveolar concentration (MAC) of inhaled anesthetic agent.120 Recent data utilizing a rodent model suggest that electroacupuncture may have beneficial effects in treatment of pain associated with bone cancer.121,122 As yet, there is no evidence that acupuncture provides pain relief in veterinary patients. Over the last few years, it has become evident that the pain transmission system is plastic (i.e., it alters in response to inputs). This plasticity results in a unique neurobiologic signature within the CNS and peripheral nervous system for each painful disease. Understanding the individual neurobiologic signatures for different disease processes should allow novel, targeted, and more effective treatments to be established.123 This approach should also allow for a more informed choice to be made regarding which of the currently available drugs might be most effective. The first relevant model of cancer pain has been established in rats—an OSA model.124 Prior to this model, evaluation of mechanisms and treatments were undertaken in chronic pain models such as sciatic nerve ligation or injection of chronic irritants—models that did not involve cancer. The introduction of clinically relevant cancer pain models is allowing tremendous progress to be made in the translation to effective human cancer pain treatment.104 It is likely that spontaneous cancer in veterinary species will play a significant role in the future in the development of new analgesic approaches for humans, with an obvious benefit to canine and feline patients. Indeed, we are starting to see this with an exciting example being the evaluation of resiniferatoxin in dogs with bone cancer.5 1. Yoxall, AT. Pain in small animals—its recognition and control. J Small Anim Pract. 1978;19:423–438. 2. Weinstein, JI, Payne, S, Poulson, JM, et al. Use of force plate analysis to evaluate the efficacy of external beam radiation to alleviate osteosarcoma pain. Vet Radiol Ultrasound. 2009;50:673–678. 3. Barnard, SM, Zuber, RM, Moore, AS. Samarium Sm 153 lexidronam for the palliative treatment of dogs with primary bone tumors: 35 cases (1999-2005). J Am Vet Med Assoc. 2007;230:1877–1881. 4. Bateman, KE, Catton, PA, Pennock, PW, et al. 0-7-21 radiation therapy for the palliation of advanced cancer in dogs. J Vet Intern Med. 1994;8:394–399. 5. Brown, DC, Iadarola, MJ, Perkowski, SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. 6. Carsten, RE, Hellyer, PW, Bachand, AM, et al. Correlations between acute radiation scores and pain scores in canine radiation patients with cancer of the forelimb. Vet Anaesth Analg. 2008;35:355–362. 7. Coomer, A, Farese, J, Milner, R, et al. Radiation therapy for canine appendicular osteosarcoma. Vet Comp Oncol. 2009;7:15–27. 8. Davis, KM, Hardie, EM, Lascelles, BD, et al. Feline fibrosarcoma: perioperative management. Compend Contin Educ Vet. 2007;29:712–714. [716–720, 722–729 passim]. 9. Fan, TM, Charney, SC, de Lorimier, LP, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2009;23:152–160. 10. Fan, TM, de Lorimier, LP, Charney, SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74–80. 11. Fan, TM, de Lorimier, LP, O’Dell-Anderson, K, et al. Single-agent pamidronate for palliative therapy of canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2007;21:431–439. 12. Karai, L, Brown, DC, Mannes, AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. 13. Knapp-Hoch, HM, Fidel, JL, Sellon, RK, et al. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 2009;45:24–32. 14. Mueller, F, Poirier, V, Melzer, K, et al. Palliative radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. IN VIVO. 2005;19:713–716. 15. Fan, TM, Barger, AM, Fredrickson, RL, et al. Investigating CXCR4 expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:602–608. 16. Fan, TM, Barger, AM, Sprandel, IT, et al. Investigating TrkA expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:1181–1188. 17. Lascelles, BDX. Surgical pain: Pathophysiology, assessment and treatment strategies. In: Tobias KM, Johnston SA, eds. Veterinary surgery small animal. St. Louis: Elsevier Saunders, 2012. 18. Marcus, DA. Epidemiology of cancer pain. Curr Pain Headache Rep. 2011;15:231–234. 19. Dohoo, SE, Dohoo, IR. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J. 1996;37:552–556. 20. Dohoo, SE, Dohoo, IR. Postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J. 1996;37:546–551. 21. Dohoo, SE, Dohoo, IR. Attitudes and concerns of Canadian animal health technologists toward postoperative pain management in dogs and cats. Can Vet J. 1998;39:491–496. 22. Watson, AD, Nicholson, A, Church, DB, et al. Use of anti-inflammatory and analgesic drugs in dogs and cats. Aust Vet J. 1996;74:203–210. 23. Williams, VM, Lascelles, BD, Robson, MC. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand. N Z Vet J. 2005;53:193–202. 24. Hugonnard, M, Leblond, A, Keroack, S, et al. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Vet Anaesth Analg. 2004;31:154–163. 25. Raekallio, M, Heinonen, KM, Kuussaari, J, et al. Pain alleviation in animals: attitudes and practices of Finnish veterinarians. Vet J. 2003;165:131–135. 26. Capner, CA, Lascelles, BD, Waterman-Pearson, AE. Current British veterinary attitudes to perioperative analgesia for dogs. Vet Rec. 1999;145:95–99. 27. Lascelles, BD, Main, DC. Surgical trauma and chronically painful conditions–within our comfort level but beyond theirs? J Am Vet Med Assoc. 2002;221:215–222. 28. Brambell FWR: Report of technical committee to enquire into the welfare of animals kept under intensive husbandry systems (Cmnd 2836). In Vol. London, HM Stationery Office, 1965. 29. Baxevanis, CN, Papilas, K, Dedoussis, GV, et al. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71:82–88. 30. Lennard, TW, Shenton, BK, Borzotta, A, et al. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72:771–776. 31. Kutza, J, Gratz, I, Afshar, M, et al. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85:918–923. 32. Oka, M, Hazama, S, Suzuki, M, et al. Depression of cytotoxicity of nonparenchymal cells in the liver after surgery. Surgery. 1994;116:877–882. 33. Sandoval, BA, Robinson, AV, Sulaiman, TT, et al. Open versus laparoscopic surgery: a comparison of natural antitumoral cellular immunity in a small animal model. Am Surg. 1996;62:625–630. [discussion 630–631]. 34. Eggermont, AM, Steller, EP, Sugarbaker, PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery. 1987;102:71–78. 35. Allendorf, JD, Bessler, M, Horvath, KD, et al. Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc. 1998;12:1035–1038. 36. Allendorf, JD, Bessler, M, Horvath, KD, et al. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13:233–235. 37. Allendorf, JD, Bessler, M, Kayton, ML, et al. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649–653. 38. Lacy, AM, Garcia-Valdecasas, JC, Delgado, S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. 39. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. 40. Lee, SW, Whelan, RL. Immunologic and oncologic implications of laparoscopic surgery: what is the latest? Clin Colon Rectal Surg. 2006;19:5–12. 41. Page, GG, Blakely, WP, Ben-Eliyahu, S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. 42. Lee, SW, Gleason, NR, Southall, JC, et al. A serum-soluble factor(s) stimulates tumor growth following laparotomy in a murine model. Surg Endosc. 2000;14:490–494. 43. Wada, H, Seki, S, Takahashi, T, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499–506. 44. Conzemius, MG, Hill, CM, Sammarco, JL, et al. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J Am Vet Med Assoc. 1997;210:1619–1622. 45. Arpinelli, F, Bamfi, F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual Life Outcomes. 2006;4:85. 46. Apolone, G, De Carli, G, Brunetti, M, et al. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. Pharmacoeconomics. 2001;19:187–195. 47. Yazbek, KV, Fantoni, DT. Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. J Am Vet Med Assoc. 2005;226:1354–1358. 48. Lynch, S, Savary-Bataille, K, Leeuw, B, et al. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet Comp Oncol. 2011;9:172–182. 49. Tzannes, S, Hammond, MF, Murphy, S, et al. Owners “perception of their cats” quality of life during COP chemotherapy for lymphoma. J Feline Med Surg. 2008;10:73–81. 50. Bergh, MS, Budsberg, SC. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med. 2005;19:633–643. 51. Papich, MG. An update on nonsteroidal anti-inflammatory drugs (NSAIDs) in small animals. Vet Clin North Am Small Anim Pract. 2008;38:1243–1266. 52. Innes, JF, Clayton, J, Lascelles, BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec. 2010;166:226–230. 53. Lascelles, BD, Court, MH, Hardie, EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg. 2007;34:228–250. 54. Kukanich, B, Bidgood, T, Knesl, O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet Anaesth Analg. 2012;39:69–90. 55. Wernham, BG, Trumpatori, B, Hash, J, et al. Dose reduction of meloxicam in dogs with osteoarthritis-associated pain and impaired mobility. J Vet Intern Med. 2011;25:1298–1305. 56. Smith, HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269–280. 57. Mburu, DN. Evaluation of the anti-inflammatory effects of a low dose of acetaminophen following surgery in dogs. J Vet Pharmacol Ther. 1991;14:109–111. 58. Mburu, DN, Mbugua, SW, Skoglund, LA, et al. Effects of paracetamol and acetylsalicylic acid on the post-operative course after experimental orthopaedic surgery in dogs. J Vet Pharmacol Ther. 1988;11:163–170. 59. Kukanich, B, Lascelles, BD, Aman, AM, et al. The effects of inhibiting cytochrome P450 3A, p-glycoprotein, and gastric acid secretion on the oral bioavailability of methadone in dogs. J Vet Pharmacol Ther. 2005;28:461–466. 60. Kukanich, B, Lascelles, BD, Papich, MG. Pharmacokinetics of morphine and plasma concentrations of morphine-6-glucuronide following morphine administration to dogs. J Vet Pharmacol Ther. 2005;28:371–376. 61. Kukanich, B, Papich, MG. Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs. J Vet Pharmacol Ther. 2004;27:337–341. 62. KuKanich, B. Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine-6-glucuronide in healthy Greyhound dogs. J Vet Pharmacol Ther. 2010;33:15–21. 63. Lascelles, BD, Robertson, SA. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats. Am J Vet Res. 2004;65:1085–1089. 64. Robertson, SA, Taylor, PM, Lascelles, BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec. 2003;153:462–465. 65. Lascelles, BD, Robertson, SA, Taylor, PM, et al. Comparison of the pharmacokinetics and thermal antinociceptive pharmacodynamics of 20mcg/kg buprenorphine administered sublingually or intravenously in cats. Vet Anaesth Analg. 2003;30:109. [(Abstr)]. 66. Woolf, CJ, Thompson, SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: implication for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. 67. Julius, D, Basbaum, AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. 68. Graven-Nielsen, T, Arendt-Nielsen, L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4:313–321. 69. Wagner, AE, Walton, JA, Hellyer, PW, et al. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. J Am Vet Med Assoc. 2002;221:72–75. 70. Slingsby, LS, Waterman-Pearson, AE. The post-operative analgesic effects of ketamine after canine ovariohysterectomy–a comparison between pre- or post-operative administration. Res Vet Sci. 2000;69:147–152. 71. Eisenberg, E, Pud, D. Can patients with chronic neuropathic pain be cured by acute administration of the NMDA receptor antagonist amantadine? Pain. 1998;74:337–339. 72. Lascelles, BD, Gaynor, JS, Smith, ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med. 2008;22:53–59. 73. Vernier, VG, Harmon, JB, Stump, JM, et al. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol. 1969;15:642–665. 74. Dayer, P, Desmeules, J, Collart, L. Pharmacology of tramadol. Drugs. 1997;53:18–24. 75. Oliva, P, Aurilio, C, Massimo, F, et al. The antinociceptive effect of tramadol in the formalin test is mediated by the serotonergic component. Eur J Pharmacol. 2002;445:179–185. 76. KuKanich, B, Papich, MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Ther. 2004;27:239–246. 77. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Pharmacokinetic and urine profile of tramadol and its major metabolites following oral immediate release capsules administration in dogs. Vet Res Commun. 2009. 78. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Pharmacokinetics of tramadol and its major metabolites following rectal and intravenous administration in dogs. N Z Vet J. 2009;57:146–152. 79. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Biopharmaceutical profile of tramadol in the dog. Vet Res Commun. 2009;33(Suppl 1):189–192. 80. Giorgi, M, Saccomanni, G, Lebkowska-Wieruszewska, B, et al. Pharmacokinetic evaluation of tramadol and its major metabolites after single oral sustained tablet administration in the dog: a pilot study. Vet J. 2009;180:253–255. 81. McMillan, CJ, Livingston, A, Clark, CR, et al. Pharmacokinetics of intravenous tramadol in dogs. Can J Vet Res. 2008;72:325–331. 82. Torring, ML, Riis, A, Christensen, S, et al. Perforated peptic ulcer and short-term mortality among tramadol users. Br J Clin Pharmacol. 2008;65:565–572. 83. Garcia-Hernandez, L, Deciga-Campos, M, Guevara-Lopez, U, et al. Co-administration of rofecoxib and tramadol results in additive or sub-additive interaction during arthritic nociception in rat. Pharmacol Biochem Behav. 2007;87:331–340. 84. Papich, MG, Bledsoe, DL. Tramadol pharmacokinetics in cats after oral administration of an immediate release tablet. J Vet Intern Med. 2007;21:616. [(abstr.)]. 85. Pypendop, BH, Ilkiw, JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther. 2008;31:52–59. 86. Brondani, JT, Luna, SP, Marcello, GC, et al. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg. 2009;11:503–509. 87. Pypendop, BH, Siao, KT, Ilkiw, JE. Effects of tramadol hydrochloride on the thermal threshold in cats. Am J Vet Res. 2009;70:1465–1470. 88. Radulovic, LL, Turck, D, von Hodenberg, A, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23:441–448. 89. Vollmer, KO, von Hodenberg, A, Kolle, EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–839. 90. Platt, SR, Adams, V, Garosi, LS, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. 2006;159:881–884. 91. Wagner, AE, Mich, PM, Uhrig, SR, et al. Clinical evaluation of perioperative administration of gabapentin as an adjunct for postoperative analgesia in dogs undergoing amputation of a forelimb. J Am Vet Med Assoc. 2010;236:751–756. 92. Siao, KT, Pypendop, BH, Ilkiw, JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res. 2010;71:817–821. 93. Pypendop, BH, Siao, KT, Ilkiw, JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res. 2010;71:1027–1032. 94. Verdu, B, Decosterd, I, Buclin, T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–2632. 95. Kautio, AL, Haanpaa, M, Leminen, A, et al. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 2009;29:2601–2606. 96. Kautio, AL, Haanpaa, M, Saarto, T, et al. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35:31–39. 97. Chew, DJ, Buffington, CA, Kendall, MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc. 1998;213:1282–1286. 98. Challapalli, V, Tremont-Lukats, IW, McNicol, ED, et al. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 4, 2005. [CD003345]. 99. Fleming, JA, O’Connor, BD. Use of lidocaine patches for neuropathic pain in a comprehensive cancer centre. Pain Res Manag. 2009;14:381–388. 100. Ko, J, Weil, A, Maxwell, L, et al. Plasma concentrations of lidocaine in dogs following lidocaine patch application. J Am Anim Hosp Assoc. 2007;43:280–283. 101. Ko, JC, Maxwell, LK, Abbo, LA, et al. Pharmacokinetics of lidocaine following the application of 5% lidocaine patches to cats. J Vet Pharmacol Ther. 2008;31:359–367. 102. Weiland, L, Croubels, S, Baert, K, et al. Pharmacokinetics of a lidocaine patch 5% in dogs. J Vet Med A Physiol Pathol Clin Med. 2006;53:34–39. 103. Weil, AB, Ko, J, Inoue, T. The use of lidocaine patches. Compend Contin Educ Vet. 2007;29:208–210. [212, 214–206]. 104. Jimenez Andrade, JM, Mantyh, P. Cancer pain: From the development of mouse models to human clinical trials. In: Kruger L, Light AR, eds. Translational pain research: from mouse to man. Boca Raton, FL: CRC Press, 2010. 105. Jimenez-Andrade, JM, Mantyh, WG, Bloom, AP, et al. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. 106. Sabino, MA, Mantyh, PW. Pathophysiology of bone cancer pain. J Support Oncol. 2005;3:15–24. 107. Milner, RJ, Farese, J, Henry, CJ, et al. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597–604. 108. Fan, TM. Intravenous aminobisphosphonates for managing complications of malignant osteolysis in companion animals. Top Companion Anim Med. 2009;24:151–156. 109. Ashton, JA, Farese, JP, Milner, RJ, et al. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005;66:885–891. 110. Farese, JP, Ashton, J, Milner, R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim. 2004;40:113–117. 111. Fan, TM, de Lorimier, LP, Garrett, LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med. 2008;22:380–387. 112. de Lorimier, LP, Fan, TM. Bone metabolic effects of single-dose zoledronate in healthy dogs. J Vet Intern Med. 2005;19:924–927. 113. Tomlin, JL, Sturgeon, C, Pead, MJ, et al. Use of the bisphosphonate drug alendronate for palliative management of osteosarcoma in two dogs. Vet Rec. 2000;147:129–132. 114. Wiley, RG, Lappi, DA. Targeted toxins in pain. Adv Drug Deliv Rev. 2003;55:1043–1054. 115. Wiley, RG. Substance P receptor-expressing dorsal horn neurons: lessons from the targeted cytotoxin, substance P-saporin. Pain. 2008;136:7–10. 116. Wiley, RG, RH, Kline, Vierck, CJ, Jr. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. 117. Allen, JW, Horais, KA, Tozier, NA, et al. Intrathecal substance P-Saporin selectively lesions NK-1 receptor bearing neurons in dogs. J Pain. 2002;3(suppl 1):51. 118. Ramirez, O, 3rd., Dodge, RK, Page, RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 1999;40:517–522. 119. Milner, RJ, Dormehl, I, Louw, WK, et al. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J S Afr Vet Assoc. 1998;69:12–17. 120. Culp, LB, Skarda, RT, Muir, WW, 3rd. Comparisons of the effects of acupuncture, electroacupuncture, and transcutaneous cranial electrical stimulation on the minimum alveolar concentration of isoflurane in dogs. Am J Vet Res. 2005;66:1364–1370. 121. Zhang, RX, Li, A, Liu, B, et al. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12:870–878. 122. Zhang, RX, Li, A, Liu, B, et al. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1 beta expression in a rat model. Anesth Analg. 2007;105:1482–1488. [table of contents]. 123. Mantyh, PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. 124. Honore, P, Menning, PM, Rogers, SD, et al. Neurochemical plasticity in persistent inflammatory pain. Prog Brain Res. 2000;129:357–363.
Supportive Care for the Cancer Patient
 Section A
Section A
Management of Chronic Cancer Pain
How Painful Is Cancer in Animals?
Tumor Category
Notes
Tumors involving bone
Primary bone tumors (both of the appendicular and axial skeleton) and metastasis to bone are painful. Just as in humans, sometimes metastasis to bone can be relatively nonpainful; however, this should be considered the exception.
Central nervous system tumors
Extradural tumors that expand and put pressure on neural tissue are often associated with pain. Tumors originating from within the neural tissue are often not associated with pain until later on in the course of the disease. In humans with primary brain tumors or metastases to brain, 60%-90% of them suffer from headaches; it should be presumed that animals also suffer such headaches.
Gastrointestinal
Especially esophagus, stomach, colon, and rectum. Such pain may be very difficult to localize, and it may manifest as vague signs and behavioral changes. Colonic and rectal pain is often manifested as perineal discomfort.
Inflammatory mammary carcinoma
This form of mammary cancer is very painful in humans, and dogs with this form of mammary cancer appear to exhibit obvious signs of pain.
Genitourinary tract tumors
Stretching of the renal capsule appears to produce significant pain. Bladder tumors appear to be predictably associated with pain. Tumors of the distal genitourinary tract are often manifested as perineal pain or pain apparently associated with the lower back.
Prostate
Prostatic tumors appear to be particularly painful, especially if local metastasis to bone is present, and the pain may be manifested as lower back or abdominal pain.
Oral and pharyngeal tumors
Soft tissue tumors that are growing by projecting from the surface (e.g., projecting from the gingival surface) appear to be relatively nonpainful. Tumors involving bone or those growing within the tissues of the maxilla or mandible appear to be significantly more painful. Soft tissue tumors of the pharynx and caudal oral cavity are particularly painful.
Intranasal tumors
Pain probably results both from the destruction of turbinates and from the destruction of bone of the nasal cavity.
Invasive soft tissue sarcomas
The aggressive vaccine-associated sarcomas in cats are particularly painful—the apparent size of the lesion does not correlate with the degree of pain. Other invasive sarcomas in both species are painful.
Also, in both cats and dogs, noninvasive soft tissue sarcomas that are pressing on nerves and other sensitive structures will be painful. One form of soft tissue sarcomas, the peripheral nerve sheath tumor (PNST), is sometimes reported to be painful to the touch.
Invasive cutaneous tumors
Especially those that are ulcerative.
Liver and biliary tumors
Especially those that are expansile, stretching the liver capsule. Expansile liver tumors are reported to be painful in humans.
Disseminated intrathoracic and intraabdominal tumors (e.g., mesothelioma, malignant histiocytosis)
The signs associated with such tumors are particularly vague; however, often, intracavitary analgesia (such as intraabdominal local anesthetic) can markedly improve the animal’s demeanor, and thus, just as in humans, it appears disseminated neoplasia of these cavities is associated with significant pain.
Lung tumors
Although significant pain is reported in humans with lung cancer, often animals appear to show few signs of pain. However, even in those animals, the provision of an analgesic can often improve demeanor.
Pain following surgical removal of a tumor
Pain well beyond the postoperative period occurs in animals that have undergone surgery and is probably neuropathic in nature. Phantom pain (such as phantom limb pain), a form of neuropathic pain, does appear to exist in animals. If tumor recurrence occurs, significant pain is usually associated with this.
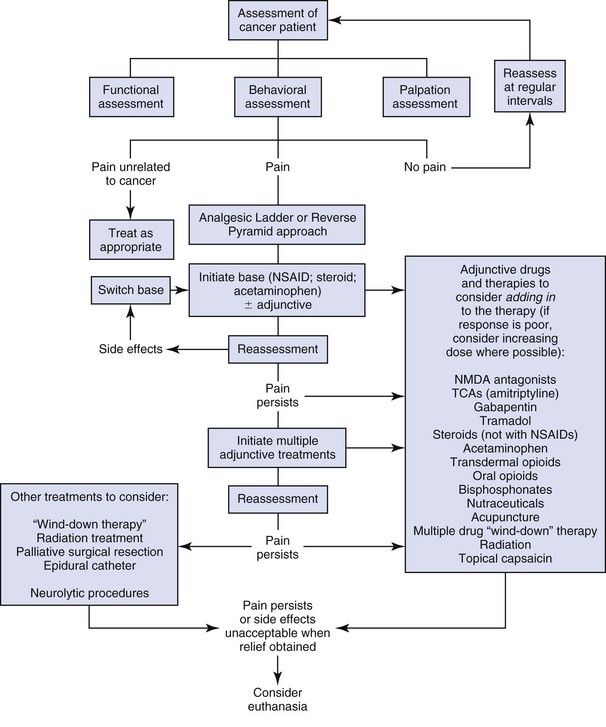
General Approach to Cancer Pain Management
A
Assess the patient
Ask for the owner’s perceptions of the pain present or of any compromise of the animal’s quality of life. Assess the patient thoroughly, using palpation and observation.
B
Believe the owner
The owner sees the pet every day in its own environment and knows when alterations in behavior occur. They can rarely suggest diagnoses, but they do know when something is wrong and when their pet is in pain, just as a mother knows when something is wrong with her child.
C
Choose appropriate therapy
Anything other than mild pain should be treated with more than one class of analgesic, or an analgesic drug combined with nondrug adjunctive therapy. Be aware of potential drug interactions.
D
Deliver therapy
Deliver the therapy in a logical coordinated manner and explain carefully to the owner about any possible side effects.
E
Empower the client
Empower the clients to participate actively in their pet’s treatment; ask for feedback and updates on how the therapy is working.
The Importance of Alleviating Pain in the Cancer Patient
Assessment of Cancer Pain
Behavior
Notes
Activity
Less activity than normal; may be very specific activities that are changed; decreased jumping; less playing; less venturing outside; less willing to go on walks (dogs); stiff gait, altered gait, or lameness can be associated with generalized pain but is more often associated with limb or joint pain; slow to rise and get moving after rest (osteoarthritis is often concurrently present).
Appetite
Often decreased with chronic pain.
Attitude
Any change in behavior can be associated with cancer pain—aggressiveness, dullness, shyness, “clinginess,” increased dependence
Facial expression
Head hung low, squinted eyes in cats. Sad expression in dogs, head carried low.
Grooming
Failure to groom can be due to either a painful oral lesion or generalized pain.
Response to palpation
This is one of the best ways to diagnose and monitor pain. Pain can be elicited by palpation of the affected area, or manipulation of the affected area, which exacerbates the pain present. This is manifested as an aversion response from the animal (i.e., the animal attempts to escape the procedure, or yowls, cries, hisses, or bites). Pain is inferred when this occurs.
Respiration
May be elevated with severe cancer pain.
Self-trauma
Licking at an area (e.g., joint with osteoarthritis, bone with primary bone cancer, the abdomen with intraabdominal cancer) can indicate pain; scratching can indicate pain (e.g., scratching at cutaneous tumors, scratching and biting at the flank with prostatic or colonic neoplasia).
Urinary and bowel elimination
Failure to use litter box (cats); urinating and defecating inside (dogs).
Vocalization
Vocalization is rare in response to chronic pain in dogs and cats; however, owners of dogs will often report frequent odd noises (whining, grunting) associated with cancer pain. Occasionally, cats will hiss, utter spontaneous plaintive meows, or purr in association with cancer pain
Principles of Alleviation of Cancer Pain
Classification of Cancer Pain
Drug
Dose for Dogs
Comments
Paracetamol (acetaminophen)
10-15 mg/kg PO q12 hrs
Associated with fewer GI side effects than regular NSAIDs and has not been noted to be associated with renal toxicity. Toxicity has, however, not been evaluated clinically in dogs. Can be combined with regular NSAIDs in severe cancer pain, but this combination has not been evaluated for toxicity.
Paracetamol (acetaminophen) + codeine (30 or 60 mg)
10-15 mg/kg of acetaminophen
Sedation can be seen as a side effect with doses at or above 2 mg/kg codeine.
Amantadine
4.0-5.0 mg/kg PO q24 hrs
Loose stools and excess GI gas can be seen at higher doses for a few days. Should not be combined with drugs such as selegiline or sertraline until more is known about drug interactions. Should not be used in seizure patients or patients in heart failure.
Amitriptyline
0.5-2.0 mg/kg PO q24 hrs
Has not been evaluated for clinical toxicity in the dog. Should be used cautiously in combination with tramadol.
Butorphanol
0.2-0.5 mg/kg PO up to q8 hrs
May produce sedation at higher doses. Not a very predictable analgesic, especially in the dog, and best when used in combination with other analgesics (e.g., NSAIDs).
Codeine
0.5-2.0 mg/kg PO q8-12 hrs
Sedation can be seen at higher doses. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect.
Fentanyl, transdermal
2-5 mcg/kg/hr
Can be very useful in the short-term control of cancer pain. For long-term therapy, usefulness is limited due to need to change the patch every 4 to 7 days.
Gabapentin
3-10 mg/kg PO q6-12 hrs
Has not been evaluated in dogs as an analgesic. The most likely side effect is sedation.
Glucosamine and chondroitin sulfate
13-15 mg/kg chondroitin sulfate PO q24 hrs
Can be used in a variety of cancer pains due to its mild antiinflammatory and analgesic effects.
Morphine, liquid
0.2-0.5 mg/kg PO q 6-8 hrs
Can be useful for dosing smaller dogs where the morphine tablets are not suitable. Sedation and particularly constipation are side effects that are seen as the dose is increased. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect.
Morphine, sustained release
0.5-3.0 mg/kg PO q 8-12 hrs
Doses higher than 0.5-1.0 mg/kg are often associated with unacceptable constipation according to owners, so suggest using 0.5 mg/kg several times a day. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect.
Pamidronate
1-1.5 mg/kg slowly IV diluted in 4 mL/kg normal saline, administered over 2 hrs; repeat every 4-6 weeks
Inhibits osteoclast activity and thus only provides analgesia in cases suffering from a primary or metastatic bone tumor that is causing osteolysis. Effect may be delayed days-weeks.
Prednisolone
0.25-1 mg/kg PO q12-24 hrs; taper to q48 hrs if possible after 14 days
Do NOT use concurrently with NSAIDs. Can be particularly useful in providing analgesia when there is a significant inflammatory component associated with the tumor and for CNS or nerve tumors.
Tramadol
4-5 mg/kg PO q6-12 hrs
This drug has not been evaluated for efficacy or toxicity in dogs.
Drug
Cat Dose (mg/kg)
Notes
Acetaminophen (Paracetamol)
Contraindicated
Small doses rapidly cause death in cats.
Amantadine
3.0-5.0 mg/kg PO q24 hrs
This drug has not been evaluated for toxicity but is well tolerated in dogs and humans, with occasional side effects of agitation and GI irritation. May be a useful addition to NSAIDs in the treatment of chronic cancer pain conditions. Amantadine powder can be purchased and formulated into appropriately sized capsules. The kinetics have recently been evaluated in cats.
Amitriptyline
0.5-2.0 mg/kg PO q24 hrs
This drug appears to be well tolerated for up to 12 months of daily administration. May be a useful addition to NSAIDs for treatment of chronic pain conditions.
Aspirin
10 mg/kg PO q48 hrs
Can cause significant GI ulceration.
Buprenorphine
0.01-0.02 mg/kg SL q8-12 hrs
SL route is not resented by cats and may be a good way to provide postoperative analgesia at home. Feedback from owners indicates that after 2-3 days dosing at this dose, anorexia develops. Smaller doses (5-10 µg/kg) may be more appropriate for “long-term” administration, especially in combination with other drugs.
Butorphanol
0.2-1.0 mg/kg PO q6 hrs
One study suggests using oral form after surgery may be beneficial. Generally considered to be a poor analgesic in cats except for visceral pain; however, the author has found it to be useful as part of a multimodal approach to cancer pain therapy.
Carprofen
Not enough data to enable recommendations for long-term administration.
—
Etodolac
Not recommended.
—
Firocoxib
—
Has not been reported in clinical cases, but it has a half-life of 8-12 hours in the cat, and at 3 mg/kg provided antipyretic effects in a pyrexia model.
Flunixin meglumine
1 mg/kg PO daily for 7 days
Daily dosing for 7 days results in increased rate of metabolism of the drug but a rise in liver enzymes, suggesting liver toxicity may be a problem with prolonged dosing.
Gabapentin
10 mg/kg q12 hrs
Appears to be particularly effective in chronic pain in cats where an increase in sensitivity has occurred, or where the pain appears to be excessive in comparison to the lesion present.
Glucosamine/chondroitin sulphate combinations
Approximately 15 mg/kg chondroitin sulphate PO q12-24 hrs
May be associated with mild analgesic effects.
Glucosamine/chondroitin sulphate combination with avocado/soya extracts
Labelled dose
May be associated with mild analgesic effects.
Ketoprofen
1 mg/kg PO q24 hrs
Probably well tolerated as pulse therapy for chronic pain, with a few days “rest” between treatments of approximately 5 days. Has also been used by some at 1 mg/kg every 3 days long term. Another approach has been to use 0.5 mg/kg daily for 5 days (weekdays) followed by no drug over the weekend and repeated.
Meloxicam
0.01 mg/kg PO on day 1, followed by 0.05 mg/kg PO daily for 4 days, then 0.05 mg/kg every other day thereafter. (Approval in the European Union at 0.05mg/kg daily indefinitely for musculoskeletal pain.)
Well received by cats due to its formulation as a honey syrup. Also, the drop formulation makes it very easy to gradually and accurately decrease the dose. A decreasing regimen has not been evaluated for efficacy in cats but has been found to be successful in dogs. Meloxicam should be dosed accurately using syringes.
Morphine (oral liquid)
0.2-0.5 mg/kg PO tid-qid
Best compounded into a palatable, flavored syrup; however, cats usually resent this medication. Morphine may not be as effective in cats as it is in dogs.
Morphine (oral sustained release)
Tablets too large for dosing cats.
—
Piroxicam
1 mg/cat PO daily for a maximum of 7 days. If longer term medication is considered, suggest every other day dosing.
Daily dosing for 7 days results in a slight increase in the half-life.
Prednisolone
0.5-1.0 mg/kg PO q24 hrs
Can be very effective. NOT to be combined with concurrent NSAID administration.
Polysulfated glycosaminoglycans (PSGAGs; Adequan)
5 mg/kg SQ twice weekly for 4 weeks; then once weekly for 4 weeks; then once monthly (other suggested regimens call for once weekly injections for 4 weeks, then once monthly).
There is no evidence-based medicine that it provides any analgesic effect, but anecdotal information suggests improvement can be seen after a few injections.
Robenacoxib
1-2 mg/kg q24 hrs
Has varying approvals in different parts of the world (approved for up to 11 days administration in Switzerland). First NSAID that is a COX-2 inhibitor, has a short half-life, and demonstrates tissue selectivity.
Tepoxalin
5-10 mg/kg q24 hrs
The author has used this successfully long-term in cats, likely due to its short half-life (5 hours) and true dual inhibition characteristics.
Tolfenamic acid
4 mg/kg PO q24 hrs for 3 days maximum
Has not been evaluated for chronic pain, but recent objective measurements demonstrate analgesia in the cat when administered perioperatively.
Tramadol
1-2 mg/kg once to twice daily
Main problem is dosing cats—the tablets are very bitter and aversive to cats.
Transdermal fentanyl patch
2-5 µg/kg/hrs
The patches may provide 5-7 days of analgesia in some cases and should be left on for longer than 3 days. Following removal, the decay in plasma levels following patch removal is slow.
Vedaprofen
0.5 mg/kg q 24 hrs for 3 days
Has not been evaluated for chronic pain but was evaluated for controlling pyrexia in upper respiratory infection, and for controlling postoperative pain following ovariohysterectomy.
Drugs and Strategies Used for Management of Pain in Cancer Patients
Nonsteroidal Antiinflammatory Drugs
Acetaminophen
Opioids
N-Methyl D-Aspartate Antagonists
Combination Analgesics
Anticonvulsant Drugs
Tricyclic Antidepressants
Sodium Channel Blockade
Bisphosphonates
Neuronal Ablation or Exhaustion Therapy
Radiation Therapy
Acupuncture
The Future: Toward a Mechanistic Understanding of Cancer Pain
References
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Supportive Care for the Cancer Patient