Ludovica Dragone, Kristinn Heinrichs, David Levine, Tyler Tucker and Darryl Millis Superficial heat and cold have been used for centuries to manage soft tissue and joint injuries with the specific goals of relieving pain, altering the physiologic processes underlying tissue healing, and affecting the elasticity of connective tissue, including muscle, tendon, ligament, and joint capsule.1 The primary goal of any thermal modality is to facilitate the ultimate therapeutic modality of exercise. A dog adjusts its temperature based on the information supplied by the peripheral receptors, and, based upon the information, activates mechanisms such as trembling, sweating, and modifying the local circulation. Thermotherapy works on this latter process.1 Thermotherapy applications may be simple or sophisticated, being delivered through hot or cold water, hot or cold packs, hot or cold whirlpools, ice massage, contrast baths, cold compression units, and other methods.1 Thermotherapy applications fall in the infrared portion of the electromagnetic spectrum just beyond the wavelength and frequency ranges for visible light.2 The shorter the wavelength, the greater the frequency and the more shallow the depth of penetration. The thermal electrophysical modalities discussed in this chapter fall within the category of “near infrared” and possess shorter wavelengths than electrical stimulating currents. Therefore, infrared electrophysical agents penetrate to a shallower depth compared with electrical stimulating currents. The biologic effects of electromagnetic radiation depend on the frequency used, duration of exposure, tissue characteristics, and power density.2 Electromagnetic energy and its transmission through the body are governed by several physical laws. The first law is the Arndt-Schultz Principle1: Tissue must absorb the energy produced by the thermal agent to stimulate the tissue’s normal function. If the energy absorbed is insufficient to stimulate the tissue, there is no effect. If too much energy is absorbed, tissue damage may occur. The second law, related to the first, is the Law of Grotthus-Draper, which determines the fate of the energy. If a tissue does not absorb the energy, it is transmitted to deeper tissue layers. If the energy is absorbed more superficially, less energy is transmitted to deeper layers and less penetration of the energy occurs. When electromagnetic energy encounters the body’s tissues, it has three possible fates: it may be reflected (e.g., reflected from the skin’s surface), it may be refracted (e.g., at the interface between the dermis and subcutaneous fat), or it may be absorbed (e.g., by muscle). The estimated depth of penetration for most infrared thermal modalities (cold or hot packs, whirlpools, paraffin baths, or luminous infrared devices) is approximately 1 to 2 cm. Heat may be transferred by the following mechanisms: • Conduction—the transfer of heat by the direct interaction of the molecules in one area with those in another area (assuming the areas have a temperature difference). (Example: cold/hot packs) • Convection—heat transfer by movement of air or fluid from a warm area to a cooler area. (Example: the circulatory system, air temperature) • Radiation—the exchange of electromagnetic energy that occurs when there is a difference in temperature between two objects. Heat transfers from a warmer to cooler area. (Example: sun, infrared lamps) • Evaporation—vapocoolant sprays are used to cool the skin and underlying tissues by causing evaporation and extracting heat from the tissues. • Conversion—the conversion of a nonthermal form of energy (mechanical, chemical) that penetrates the tissues and is converted to heat. (Example: therapeutic ultrasound; sound waves being converted to thermal energy within the tissues) Most thermal techniques transfer energy by conduction. Energy travels down a thermal gradient, with energy (heat) being removed from an object, rather than cold being added. Heat always travels from the warmer object to the cooler.3 According to Knight,4 the rate at which heat transfer occurs depends on the following factors: • The temperature difference between the body and the modality: The greater the difference, the more quickly the transfer of heat occurs. • Regeneration of body heat and modality cooling: As tissue gives up heat, the heat lost is replaced by circulating blood and surrounding tissues. Cold application tends to result in greater tissue temperature changes at greater tissue depths as compared with superficial heating modalities. For example, after a 20-minute ice pack application to the thigh muscles, the intramuscular temperature declined more slowly than the subcutaneous temperature, and there was less cooling at deeper tissue levels (and never reached as cold a temperature as the subcutaneous tissue). When the ice pack was removed the subcutaneous tissue temperature rose steeply, whereas the intramuscular temperature continued to decline for a period of time, possibly because heat was transferred from the deeper tissues to warm the superficial tissues, resulting in continued tissue temperature decreases at the greater tissue depths (Figure 18-1). • The heat storage capacity of the modality: The more energy it takes to convert a solid to a liquid (e.g., ice to water), the greater its latent heat of fusion and its capacity to remove heat from a tissue. For example crushed ice packs require more energy for this conversion to occur as compared with semisolid gel packs. Therefore ice packs cool tissues for a longer time and to a greater extent than gel packs. • The size of the modality: The larger the modality (e.g., large cold pack), the greater the energy storage capacity. • The area of the body in contact with the modality: For example cold water immersion results in the greatest tissue temperature decline compared to other methods of cold delivery because a greater surface area is in contact with the cooling modality.5 Vapocoolant sprays result in the least temperature decline and provide only superficial cooling because of the relatively small surface area cooled. • The application duration: The longer the contact time between the two surfaces, the more opportunity for energy exchange to occur. • Individual variability: Individuals with lower body fat percentage exchange heat more quickly than those with greater amounts of subcutaneous fat. One of the oldest methods of physical therapy is cryotherapy, the application of cold after trauma or surgery. Cryotherapy is used not only during the acute phase of tissue injury and healing to mitigate the effects and sequelae of tissue injury, but also after exercise during rehabilitation to minimize adverse secondary inflammatory responses.5–7 The most important physiologic effects are: The decrease in blood flow slows edema formation after injury or after surgery. The cooling of the tissue reduces the metabolic rate of the treated tissue, decreases the rate of reactions related to the acute inflammatory process, causes the inhibition of enzymatic effects related to inflammation and minimizes the release of histamine, which reduces the tissue damage.6,7 At a temperature of 30° C or lower, cartilage-degrading enzymes (protease, hyaluronidase, collagenase) are inhibited. The vasoconstriction of blood vessels also may help to decrease pain by decreasing pressure on nociceptors, which may be stimulated as a result of increased tissue swelling and edema, as well as by other physiologic means such as decreased nerve conduction velocity. The primary effect of cryotherapy is the vasoconstriction mediated by a local reflex and the central nervous system. The resulting reduced blood flow is useful not only to decrease swelling, but to control hemorrhage and the flow of inflammatory cells as well. The Hunting reaction, first described by Lewis in 1930, refers to the cyclical temperature oscillation of 2° to 6° C every 8 to 15 minutes after the tissue temperature approaches 2° C. This effect begins 20 to 40 minutes after cryotherapy application. He attributed these oscillations to cold-induced vasodilation (CIVD) as an attempt to protect tissues from cold-induced damage. However, his results have been incorrectly and widely applied to the sports medicine literature in an attempt to explain the success of cryokinetics.1 Knight et al.8 replicated Lewis’ experiment and concluded that Lewis’ observation during cold immersion and subsequent rapid increase in temperature after cryotherapy are caused by temperature effects, not CIVD and subsequent increased blood flow. Based on these studies, the primary benefits of cryotherapy are twofold: decrease in cellular metabolism and analgesic effects to permit cryoexercise to facilitate rehabilitation.4,8 The analgesic response to cryotherapy treatment is mediated by the peripheral nervous system. Peripheral thermal receptors are categorized as cutaneous myelinated type III or A delta, which are sensitive to pricking or sharp pain and cold; unmyelinated type IV or C receptors for aching pain; and unmyelinated type IV or C cutaneous receptors for pain and temperature (Table 18-1). Table 18-1 Sensory Receptors: Anatomy, Location, and Function From Guyton AC: Sensory receptors and their basic mechanisms of action. In Textbook of medical physiology, Philadelphia, 1986, Saunders. The unmyelinated cutaneous receptors respond to absolute temperature and the rate of temperature change.9 The cold-sensitive receptors begin firing at 36° C and increase their firing rate until reaching their maximum at approximately 25° C. The firing frequency drops sharply at temperatures below 20° C and is minimal by the time the receptors are cooled to 10° to 12° C. Conversely, the warm receptors also begin firing at 33° to 36° C, rapidly reach their maximum firing frequency at 43° C, and drop to a minimal firing rate at 45° C.9,10 Both the hot and cold receptors rapidly adapt to temperature changes. Central thermosensitive neurons in the preoptic and anterior hypothalamus respond to temperature changes with autonomic responses to stimulate heat retention by the body, by altering cutaneous blood flow and thermoregulatory behavior such as panting. Figure 18-2 illustrates the regulation of temperature by the hypothalamus. The anterior hypothalamus also controls thermoinsensitive neurons, such as those that are sensitive to osmolarity and glucose concentration.9,11 Stimulating the posterior hypothalamus by heating does not result in the autonomic responses characteristic of the anterior hypothalamus, but behavioral responses to the heat load occur. Local warming of the medulla increases respiratory frequency. Autonomic thermoregulatory responses also take place at the spinal cord level.11 The sympathetic nervous system response to thermal load is also chemically mediated through the release of the neurotransmitters epinephrine and norepinephrine from the adrenal medulla to induce cutaneous vasoconstriction.9 A primary effect of cryotherapy is analgesia and the concomitant reduction of reflex muscle spasm. This may be caused by the decreased nerve conduction velocity that occurs when nerves are cooled. This relationship, known as the Q10 effect, is thought to be linear until 10° C, when neural transmission is blocked.12,13 Nerve cooling also increases the duration of the refractory period, the time when a nerve cannot be stimulated by a second impulse.14 Conversely raising the subcutaneous tissue temperature increases sensory nerve conduction velocity, with the greatest effect occurring during the initial 2° C temperature elevation.15 Another mechanism postulated for the analgesic effect afforded by cryotherapy is that the cold receptors are overstimulated by cryotherapy, resulting in pain control at the spinal level by preventing pain transmission to higher centers via the spinal gate control theory of pain transmission. Cryotherapy decreases muscle spasm through a number of possible mechanisms. Muscle spasm compromises venous return and promotes lactate accumulation and acidosis. The muscle spindle receptors and Golgi tendon organ receptors fire more slowly when cooled, although the effect is less pronounced on the Golgi tendon organs.16 Sudden cooling has an excitatory effect on the muscle spindle, resulting in increased alpha motoneuron activity and increased muscle guarding. As the temperature continues to decrease, primary spindle afferent activity diminishes.12 Muscle spasm, resulting from the pain-spasm-pain cycle, stimulates the static stretch response of the type II tissue afferents. Applying superficial heat until the temperature is above 42° C decreases the muscle spindle discharge rate while it increases the firing rate of the Golgi tendon organ.16 This sequence of events results in a decrease in the firing rate of the alpha motor neuron. Thus cold may raise the threshold stimulus for muscle spindle activity, decreasing muscle spasm.17 Thermal effects on strength and endurance have also been observed by a number of authors. The force-velocity curve of muscle contraction shifts downward with decreasing temperature.18 For a given concentric contraction velocity, cooler muscles have a lower force output. Interestingly, some studies suggest that proprioception, joint position, and balance remain largely unaffected by cryotherapy, whereas others indicate that joint position awareness is affected by joint cooling.19–21 Although most authors agree that changes occur in muscle torque production with cooling, it is less clear whether those effects are specific to a particular contraction mode (concentric acceleration or eccentric deceleration). One group of researchers reported that eccentric muscle action is augmented following cryotherapy,22 whereas another group found increases in concentric, but not eccentric, torque.23 Still others found no effects on peak torque, but increased muscle endurance following cooling.24 Functionally, vertical jump performance, an indicator of lower extremity explosive power, is adversely affected by cryotherapy.25,26 These findings suggest that caution must be used in making recommendations for performance following cryotherapy. Clearly the literature on this subject is undecided as to the specific effects of cold (both immediate and delayed) on muscle strength. These findings have the most implication for dogs who are engaged in work or competitive athletic pursuits. It also requires that the clinician understand the physiology and biomechanics of muscle actions and sport demands. Cold and exercise are an effective combination in the treatment of acute musculoskeletal dysfunction unless restrictions in flexibility are present.27,28 Combining movement with cold allows for relatively greater pain-free exercise and assists in muscle pump activity to reduce acute injury-related edema. Because of these effects, cold is most effective when applied during the acute phase of trauma, typically in the first 72 hours after injury or surgery. Cryotherapy may also be useful in chronic pain situations such as arthritic joints. With this condition it can be used to reduce pain, edema, collagenolysis, synovial inflammation, and joint destruction.1,4,5 As a result of its analgesic effect, cryotherapy may permit decreased use of drugs for pain. This was noted in human medicine in a study examining numerous patients after anterior cruciate ligament surgery. Cryotherapy is currently used in postoperative treatment for swelling and pain, in musculoskeletal injury, muscle spasm, and after exercise to prevent edema or pain. This thermal modality may also have a significant influence on the effectiveness of laser therapy, given its effects on blood flow and the saturation of blood chromophores in the tissue. Where tissue perfusion is reduced with cryotherapy, the absorption of laser energy may be improved, so that deeper structures may receive greater laser energy. This use of cryotherapy before laser therapy treatment could be useful for large dogs in which the target tissue is deeply located.29 Cold application may be applied in several ways: cold water-circulating blankets, reusable ice packs, ice cubes wrapped in a towel, ice cups, cold immersion, cold compression devices, vapocoolant sprays, or contrast baths (Figure 18-3). Selection of the method depends on the desired effects, depth of penetration desired, stage of tissue healing, treatment area, and physiologic goals. Cryotherapy may be achieved by the following physical mechanisms: Local application of cold may increase connective tissue stiffness (sometimes with decreased tensile strength) and may temporarily increase muscle viscosity (with decreased ability to perform rapid movement). For sanitary reasons and to improve patient comfort and prevent damage by cryotherapy to the treated tissues, a thin towel or other material (e.g., towel or pillowcase) is commonly placed between the cold source and the skin. It is very important to observe the skin for response to cold during the treatment. A normal response at the end of a cryotherapy treatment is reddening of the skin, and an abnormal response indicating that damage may have occurred is whitening or blanching of the skin. Because of skin pigmentation in some dogs, these color changes may not be apparent, so it may be better to err on the side of caution and reduce the length of application time if there is any question. The expected changes in sensation during a treatment follow the acronym IBAN: intense burning, aching, and numbness.8 Cryotherapy is a superficial treatment, but effects of up to 1 to 4 cm of tissue depth may be noted. The depth depends on the amount of adipose tissue and local blood flow. For these reasons, cryotherapy may be more effective in the distal area of the limbs where joint capsules, tendons, and ligaments are more superficial and there is less adipose tissue.3,15,30 Examples of ice packs/cold packs are crushed ice placed in a moist towel; a plastic bag wrapped in a moist towel; or water and rubbing alcohol of a 3 : 1 ratio in a sealed plastic bag and placed in a freezer (this should result in a slush-type consistency). Numerous types of cold packs are available commercially (Figure 18-4). The treatment time is generally 10 to 20 minutes for an application of cryotherapy.
Superficial Thermal Modalities
The Physics of Thermotherapy
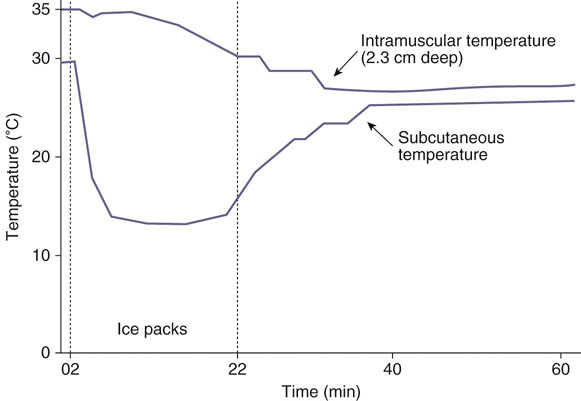
Figure 18-1 Intramuscular versus subcutaneous tissue temperature changes with cryotherapy. Intramuscular (gastrocnemius) and subcutaneous temperatures during and after a 20-minute ice pack application. Note that intramuscular temperature declined much more slowly than subcutaneous temperature and continued to decrease after the ice pack was removed. (Redrawn from Hartviksen K: Ice therapy in spasticity. Acta Neurol Scand 38(suppl 3):79-84, 1962.)
Cryotherapy
Physiologic Effects of Cryotherapy
Receptor
Type
Sensory Nerve Classification
Location
Diameter (µm)
Conduction Velocity (m/sec)
Sensory Function
Sensory (afferent) myelinated
A
Ia
Muscle
12-20
72-120
Muscle spindle primary ending (or 1-degree ending)
A
Ib
Tendon
12-20
72-120
Golgi tendon organ
Rate of muscle shortening
A
II
Muscle
6-12
36-72
Muscle spindle, secondary ending (or 2-degree ending) muscle length changes
A
II
Skin
6-12
36-72
Vibration, discriminatory touch, pacinian corpuscles
A
III
Skin
1-6
6-36
Pricking, sharp pain, temperature (cold), light touch
Unmyelinated
C
IV
Muscle
~1
0.5-2.0
Aching pain
C
IV
Skin
~1
0.5-2.0
Pain, temperature
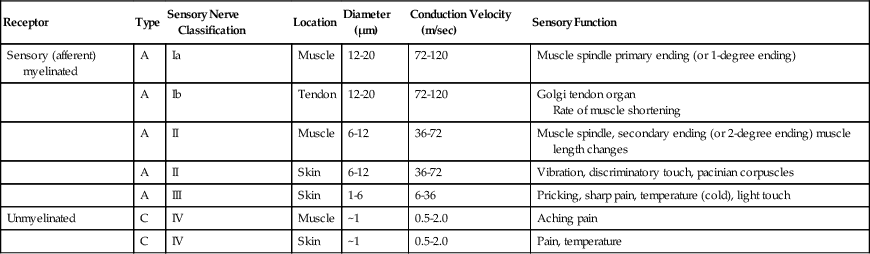
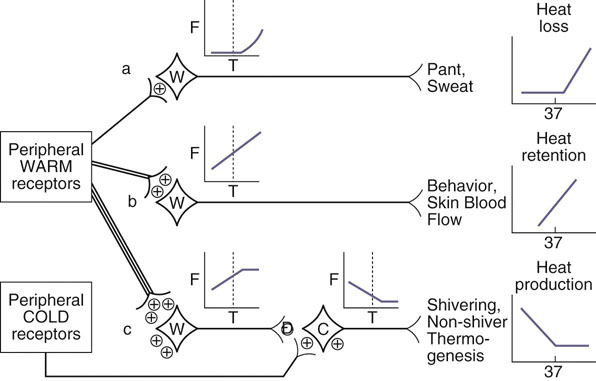
Figure 18-2 Central hypothalamic control of thermoregulation. Diagram shows neuronal control of various thermoregulatory responses. +, excitatory input; −, inhibitory input; dashed line, thermoneutral temperature; C, cold sensitive neuron; F, neuronal firing rate; T, hypothalamic temperature; W, warm sensitive neuron. (Redrawn fro Bouland JA, Dean JB: Temperature receptors in the central nervous system, Ann Rev Physiol 48:639-654, 1986.)
Cryotherapy Applications

Figure 18-3 Cryotherapy options (clockwise starting top right): Popsicle-type molds for ice massage, alcohol-water slush plastic bags, cotton wraps, commercial cold wraps, fluoromethane cold spray.
Ice Packs/Cold Packs
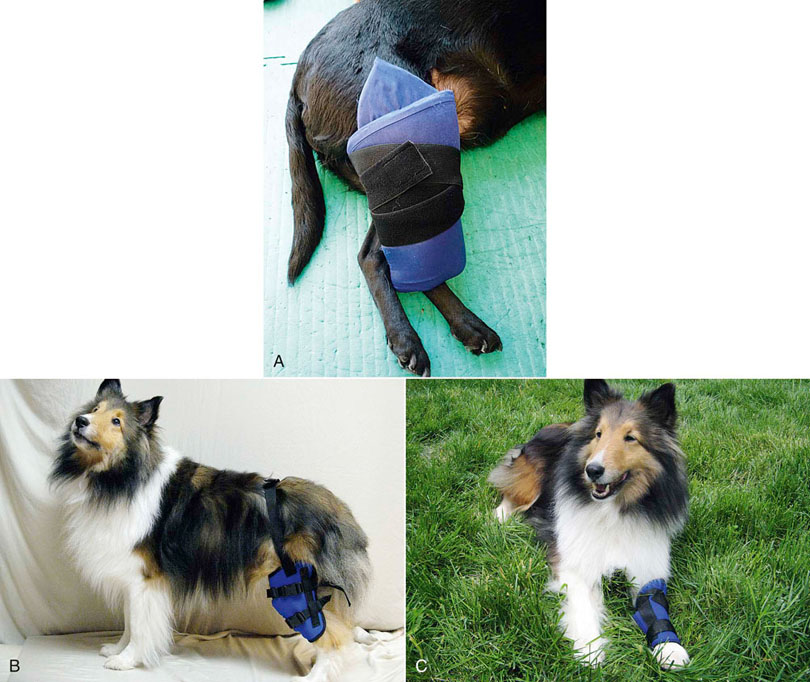
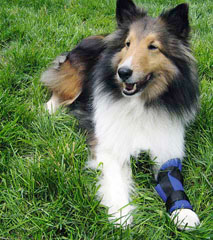
Figure 18-4 A, Example of a cryotherapy treatment using a cold pack wrapped around a dog’s hind limb. B, Canine stifle icer. C, Carpal icer.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Superficial Thermal Modalities
Only gold members can continue reading. Log In or Register to continue
