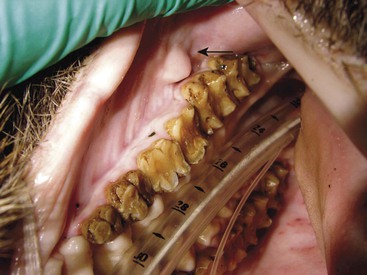
Meg Sutherland-Smith The families Suidae (swine) and Tayassuidae (peccaries) are nonruminating ungulates belonging to the Suina clade or suborder within the order Artiodactyla.49 Fossil records show suids appearing around 35 million years ago (upper Eocene era) in the part of the world now known as Thailand.49 Of the family Suidae, Suinae is the only extant subfamily. The characteristics of suid molars have resulted in dividing the subfamily into different tribes.49 The Hippohyini originated in Asia and have hypsodont dentition. Swine species from Africa with hypsodont dentition are in the Phacocherini tribe. The Suini tribe comprises members of the genus Sus. The Potamochoerini tribe includes the genera Hylochoerus and Potamochoerus. Molecular studies of Babyrousa spp. have yielded conflicting results, with some placing them in a separate tribe, Babirusina. Depending on the source, 14 to 19 species of suids exist (Table 58-1).15,32,35,49 Recent references have given species designation to the subspecies of Babyrousa babirussa.15,49 The natural range of the Suidae family spans across Europe, Africa, Asia, and East Indies. Evidence to support any wild suids having originated in the Americas is lacking. They were introduced by humans into the Americas and into Australia, New Zealand, and New Guinea. Ancestors of extant peccaries are thought to have dispersed into the New World from eastern Asia. Currently, three species of peccary range from Southwestern United States to South America (Table 58-2).15,28,35,49 TABLE 58-1 Biologic Information for Selected Species of Pigs15,32,35,49 Data modified from Morris and Shima.28 TABLE 58-2 Selected Biological Data for Peccaries14,15,28,49 Data modified from Morris and Shima.28 Wild suids and peccaries have poor vision but good hearing and a keen sense of smell. They are known for their rooting behavior. Despite their short limbs they are good runners and jumpers, and some are adept swimmers. Bornean bearded pigs have been recorded as swimming 45 kilometers (km), and babirusas have been observed swimming underwater.49 In general, wild suids and peccaries live in social groupings, although breeding males become solitary after the breeding season. Wild suid females and their offspring live in herds known as sounders. Suids are quite vocal, and peccaries make a characteristic clacking sound with their teeth. Wild suid and peccary populations are primarily threatened by habitat destruction through human encroachment and hunting.14,35,49 Isolated island populations are particularly susceptible. The International Union for the Conservation of Nature (IUCN) status of selected species is provided in Table 58-3.18 TABLE 58-3 IUCN Conservation Status of Selected Species IUCN, International Union for the Conservation of Nature. Suids are a diverse group ranging in size from 6 to 200 kilograms (kg). They are characterized as having a stout, barrel-shaped body, with a large head and short limbs relative to body size. The peccary has a piglike shape; however, the limbs are long and slender with small hooves. In addition, peccaries only have one dewclaw on the hindlimbs, and hindlimb dewclaws are generally lacking in Chacoan pecarries.49 Suid males are generally larger than females; however, little size dimorphism exists between sexes in peccaries.14,32,49 The pelage of wild suids varies from being sparse in the babirusa to being entirely covered with coarse bristly hair in the wild boar. Peccaries have a dense covering of long, coarse bristles, which makes it difficult to estimate body condition, especially during piloerection. In the genus Sus, except for Sus scrofa, all adult males have three pairs of fleshy protuberances on the face (“warts”). Warthogs also possess these characteristic facial structures. Wild suids may possess a variety of scent glands, including preputial, anal, metacarpal, mandibular, salivary, Harderian, eyelid, genal, and preorbital glands.35,49 Pecarries possess a unique dorsal rump scent gland located on the midline, approximately 15 centimeters (cm) cranial to base of the tail.14,32,49 The author has also observed a prominent papillae adjacent to the first and second maxillary molars, which are presumed to be of salivary origin in Chacoan peccaries (Figure 58-1). The suid skull is unique in that it possesses an elongated flange of bone originating from the zygomatic root referred to as the prezygomatic shelf.49 This shelf of bone separates the muscles of mastication from the muscles involved in snout movement. This adaptation is thought to facilitate rooting behavior. The disklike shape of the snout, the terminally placed nostrils, which are capable of closing, and the presence of a prenasal bone within the cartilaginous disk of the snout are also adaptions for rooting behavior.32,35,49 The prenasal bone can be seen radiographically. Babirusas tend to root in soft, moist soils, hence their rostral bone is not as well developed as in other suids or is absent.35,49 The rostrum of the Chacoan peccary is reported to have a more complex internal anatomy compared with other peccaries, and this is theorized to be an adaptation to living in dusty, xeric conditions.49 Another distinguishing feature of wild suids is their large canines. The orientation of canines in several species allows these teeth to grow upward and outward and thus capable of inflicting serious damage. In most species, the tusks of the males are larger than those of the females except in the warthog, in which both sexes have large tusks. The babirusa is known for its peculiar tusk arrangement. The alveoli of the upper canines rotate during development such that these teeth grow upward through the rostrum and spiral caudally.49 The lower canines also grow in a spiral shape. Canines are markedly reduced or absent in female babirusas. In peccaries, canines point straight down, which allows interlocking. This arrangement facilitates stabilization of the jaw when the animal is cracking hard seeds between the other teeth.14,49 The dentition of suids has been used in their taxonomic classfication.49 The desert warthog differs from other suids but is similar to other ungulates in that it does not have upper incisors. Smaller species of pigs have molars with high pointed cusps; these species tend to forage on forest vegetation and fruit, whereas larger species have dentition better suited for tougher forages, with thick enamel and conical premolars.49 Similarly, because their natural diet is composed primarily of cactus, Chacoan peccaries have higher-crowned molars versus the lower-crowned molars of the other peccary species.32,49 Except for the babirusa, suids have a simple stomach. The stomach of the babirusa is bigger and has a large diverticulum.22,32,35,49 The pH in a large area of mucus-producing cardiac glands (5.3–6.4) is able to support microbial fermentation. 22,23 Hence the babirusa is characterized as a nonruminant foregut fermenting frugivore and concentrate selector.22–24 The ultrastructure of the babirusa stomach has been extensively studied.23 Foregut fermentation is also reported in peccaries, which have a four-chambered stomach: two nonglandular blind sacs, a nonglandular gastric pouch, and a glandular hind stomach.14,32,49 Unlike pigs, peccaries do not have a gallbladder.14 The natural behaviors of wild suids and peccaries should be taken into account for appropriate exhibit specifications. The opportunity to root and dig should be provided without disrupting the structural integrity of the enclosure. These behaviors may also result in damage to indoor facilities such as padded barn floors. Animals should be given access to mud wallows, where appropriate, and kept free from fecal and urine contamination. Nesting is a common behavior, and animals should be provided bedding materials for nesting. Other considerations for housing suids and peccaries include escape potential, substrate, and intraspecific aggression. Pigs are quite capable of either jumping (both vertically and horizontally) or digging their way out of an enclosure if given the opportunity. Substrate should not be abrasive as running, pacing, or both may cause excessive hoof wear, and enclosures should be evaluated for other potential sources of foot trauma. When running along chainlink fence lines animals may traumatize the lateral dewclaws. Usually, a dominance hierarchy in social groupings exists, hence visual barriers to help divert aggression are recommended. Animals should have access to shade and, depending on the climate, protection from harsh weather. A recent study from Brazil evaluated enclosures of three different sizes for housing collared peccaries.30 Behavioral observations were performed, and the authors concluded that a minimum of approximately 200 square meters (m2) per animal of space resulted in the least agonistic behaviors. They also found that shelter use increased with allocation of more space, which supported earlier findings regarding the importance of shelters in peccary husbandry.43 Pigs are considered fourth in intelligence of animals behind primates (human and nonhuman), dolphins, and elephants; therefore they require a stimulating environment.49 They are quick learners and have sophisticated problem-solving abilities. Environmental and behavioral enrichment should be part of any program managing suids or peccaries. Many useful resources for enrichment are available.10,47 In general, wild suids and peccaries, which are considered omnivores, consume such things as leaves, grasses, young saplings, seeds, roots, tubers, fruits, fungi, eggs, invertebrates, carrion, and small vertebrates.14,32,35,49 In the wild, cactus also makes up a significant portion of the diet for the Chacoan peccary and, to a lesser extent, the white-lipped peccary.14,49 The natural biology of the various species also reveals the opportunistic nature of pig foraging based on seasonal availability.24 Some authors have considered wild suids more herbivorous, with species occupying nutritional niches.8,24 Warthogs have been classified as grazers and forest hogs as browsers. Hylochoerus, Potomachoerus sp., Sus scrofa, Sus barbatus, and possibly the warty pigs have been considered more frugivorous.8,24 The exact nutritional requirements of wild suids are not well-defined.24 In general, diets fed to wild suids and peccaries in captivity consist of a complete pelleted herbivore ration, with varying amounts of fruits, vegetables, browse, and hay. A study evaluated the apparent digestibility of different macronutrients in warthogs, red river hogs, warty pigs, and babirusa.8 No difference in protein digestibility was observed between the species, including peccaries when comparing with prior literature. Despite differences in gastric anatomy, neither peccaries nor babirusa had more efficient fiber digestion. Hemicellulose was digested more efficiently than cellulose by red river hogs, babirusas, and peccaries, whereas warthogs were capable of efficiently digesting both hemicellulose and cellulose. Therefore, dietary items high in hemicellulose would be appropriate for most wild suids, whereas incorporating items such as grass hays that have higher cellulose content would be appropriate for warthogs. In captivity, wild suids are prone to obesity, which may interfere with reproduction and exacerbate musculoskeletal conditions such as osteoarthritis. Routine weight monitoring is recommended. Feeding strategies should be incorporated that minimize the impact of social domination by one individual or a few and food-motivated aggression. In general, physical restraint is not recommended in wild suids or peccaries. Most individuals struggle violently, and no part of the body is easily held. A cornered pig may become quite aggressive, and its tusks are capable of inflicting significant injury. Attempting to restrain a nondomestic pig by the hind leg, as is done with domestic swine, is not recommended because it may result in injury to the animal. Some individual animals will remain very flighty in captivity, whereas others become quite tractable. Operant conditioning in these animals may facilitate close visual inspection and limited palpation. Some animals go into lateral recumbency when scratched with a broom or scrub brush. In animals with formidable tusks, such evaluations should be done in a protected contact situation. Piglets and infant peccaries may be manually restrained for minor procedures. Although immobilization in suids may be challenging, it is recommended for a thorough examination and for diagnostic procedures. Most wild suids have a thick layer of subcutaneous fat. Deposition of immobilizing agents into this fat layer may interfere with drug absorption, which would create a less than ideal immobilization. Peccaries generally do not have large subcutaneous fat reserves.14,32 Some animals may become quite excited during immobilization. Although exotic species do not have the genetic defect that causes malignant hyperthermia in domestic swine, they are quite susceptible to hyperthermia because of their inability to sweat and the likelihood of extreme muscle exertion during an immobilization, especially in escape scenarios.27 Excessive running, lengthy inductions, and violent recoveries are also risk factors for exertional myopathy. Foot trauma may also occur with excessive running on hard surfaces. When darting an animal in a group, precautions need to be taken to avoid causing trauma to conspecifics. In addition to remote delivery via dart systems, squeeze chutes or crates may be used for hand injections. Figure 58-2 shows a metal squeeze crate used for hand injections in small- to medium-sized animals at the author’s institution. Peccaries, warty pigs, and medium-sized red river hogs are transferred into the squeeze apparatus from a transport crate. This system works well and keeps animals confined during induction. Fasting times of 12 to 24 hours have been recommended; however, hypoglycemia has been observed in some fasted pigs, especially in Potamochoerus sp. and Sus celebifrons, at the author’s institution.5,26,28,33,48 Therefore, fasting times have been reduced to 3 to 6 hours for all suids and peccaries. In addition, blood glucose is monitored during immobilization and hypoglycemia treated, as needed. Problems secondary to a short fasting interval, for example, vomiting or gas distension of the gastrointestinal tract, have not been encountered. Chemical immobilization protocols for swine and peccaries have been reviewed recently (Table 58-4).33 Multiple drug combinations have been used successfully. In addition, the author has added ketamine at 0.5 to 1 milligram per kilogram (mg/kg) with or without azaperone (0.25–1.3 mg/kg) to medetomidine–butorphanol–midazolam combinations. This has helped minimize some of the unpredictability in the response of wild suids to immobilizing agents. Azaperone (0.5–1.5 mg/kg, intramuscularly [IM]) has also been administered to anesthetized swine prior to recovery from anesthesia in case of concern about a possible violent recovery or excitability in the postrecovery period. Attempts to handle an animal before immobilizing agents have reached their full effect or the need for additional dosing in an animal may result in significant stimulation, which may override the drug effects and prolong induction. TABLE 58-4 Reference Range for Hematologic Parameters (Adults) in Selected Species
Suidae and Tayassuidae (Wild Pigs, Peccaries)
Biology
Common Name
Scientific Name
Body Weight (adults, in kilograms)
Geographic Distribution
Dental Formula (incisors, canines, premolars, molars)
Longevity (years)
Buru babirusa
Babyrousa babyrussa
Up to 90
Indonesia, Buru, and Sulu Islands
2/3, 1/1, 2/2, 3/3
Up to 24
Bola Batu babirusa
Babyrousa bolabatuensis
Up to 90
Indonesia, Lembeh
2/3, 1/1, 2/2, 3/3
Up to 24
Malenge babirusa
Babyrousa togeanesis
Up to 90
Malenge Island
2/3, 1/1, 2/2, 3/3
Up to 24
North Sulawesi babirusa
Babyrousa celebensis
Up to 90
Northern Sulawesi
2/3, 1/1, 2/2, 3/3
Up to 24
Giant forest hog
Hylochoerus meinertzhageni
130–275
Congo basin, parts of west and east Africa
3/3, 1/1, 4/4, 3/3
—
Common warthog
Phacochoerus africanus
50–100
Sub-Saharan Africa
1/3, 1/1, 3/2, 3/3
12–15
Desert warthog
Phacochoerus aethiopicus
—
Eastern Ethiopia, northern Kenya, Somalia
—
—
Pygmy hog
Porcula salvanius
6–10
Bhutan, southern Nepal, northern India
3/3, 1/1, 4/4, 3/3
10–12
Red River hog
Potamochoerus porcus
50–120
Western Africa, Congo basin
3/3, 1/1, 4/4, 3/3
10–15
Bushpig
Potamochoerus larvatus
50–120
Eastern and southern Africa
3/3, 1/1, 4/4, 3/3
10–15
Wild boar
Sus scrofa
50–200
Europe, N. Africa, Asia; introduced into N. America, Australia, New Zealand, New Guinea
3/3, 1/1, 4/4, 3/3
15–20
Palawan pig
Sus ahoenobarbus
—
Philippines
—
—
Bearded pig
Sus barbatus
100–200
Malaysia, Sumatra, Borneo
3/3, 1/1, 4/4, 3/3
—
Heude pig
Sus bucculentus
—
Vietnam, Laos
—
—
Visayan warty pig
Sus cebifrons
—
Philippines
—
—
Celebes or warty pig
Sus celebensis
—
Indonesia
—
—
Oliver warty pig
Sus oliveri
—
Philippines
—
—
Philippine warty pig
Sus philippensis
—
Philippines
—
—
Javan warty pig
Sus verrucosus
Up to 185
Java, Bawean
3/3, 1/1, 4/4, 3/3
—
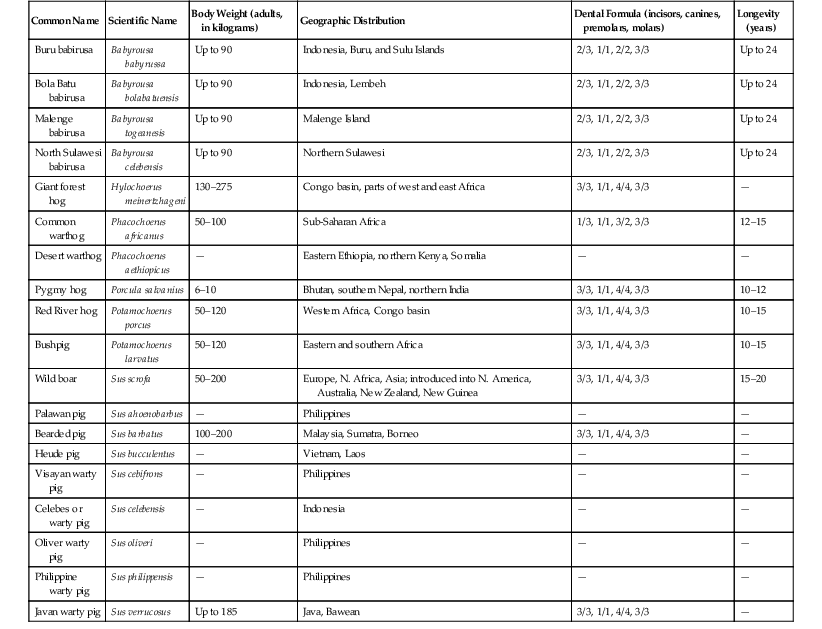
Common Name(s)
Scientific Name
Weight (adults, in kilograms)
Geographic Distribution
Dental Formula (incisors, canines, premolars, molars)
Chromosomes (2n)
Longevity (years)
Collared peccary/Javelina
Pecari tajacu
15–35
Southwest United States to Argentina
2/3, 1/1, 3/3, 3/3
30
16–24
White-lipped peccary
Tayassu pecari
27–40
Mexico to Argentina
2/3, 1/1, 3/3, 3/3
26
15–21
Chacoan peccary/Tagua
Catagonus wagneri
30–43
Argentina, Bolivia, Paraguay
2/3, 1/1, 3/3, 3/3
20
At least 9
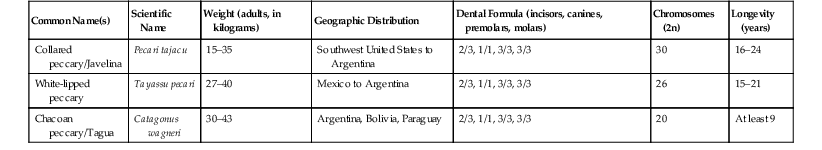
IUCN Red List Classification
Genus and Species
Critically Endangered
Porcula salvanius, Sus cebifrons
Endangered
Babyrousa togeanensis, Catagonus wagneri, Sus oliveri, Sus verrucosus
Near Threatened
Sus celebensis, Tayassu pecari
Vulnerable
Sus ahoenobarbus, Sus barbatus, Sus phillipensis
Of Least Concern
Hylochoerus meinertzhageni, Phacochoerus aethiopicus, Phacochoerus africanus, Potamochoerus larvatus, Potamochoerus porcus, Sus scofa, Pecari tajacu
Unique Anatomy
Special Housing Requirements
Feeding
Restraint and Handling
Chemical Restraint
Parameter
Red River Hog (Potamochoerus porcus)*
S. African Warthog (Phacochoerus africanus)†
Babirusa (Babyrousa babyrussa)†
Visayan Warty Pig (Sus celebrifons)*
Collared Peccary (Tayassu tajacua)†
Erythrocytes × 106/microliter (µL)
5.08–8.86
4.73–10
4.56–10.5
4.8–9.7
4.89–9.58
Packed cell volume (%)
31.3–56.5
27.4–60
28.8–54.3
30.9–53.2
26–53
Hemoglobin, gram per deciliter (g/dL)
1.36–16.2
9.4–16.2
9.1–17.6
9.9–15.9
9.4–18
Mean corpuscular volume, (dL)
17.5–69.9
39.4–71.7
51.5–93.8
25.9–69.6
43.8–92
Mean corpuscular hemoglobin, picogram (pg)
16.3–23.3
10.2–23.9
15.7–28.1
14.2–22.3
16–23.4
Mean corpuscular hemoglobin concentration, (g/dL)
28.6–36.8
25.8–36.8
26.3–35.7
0.6–33.2
21.5–36.4
Leukocytes/µL
4500–1800
4.1–15,000
3500–17,800
5900–23,300
2900–22,600
Neutrophils/µL
2745–12060
614–10,300
714–12,100
2599–18,407
1800–15,100
Band neutrophils/µL
87–424
86–801
57–595
83
44–5420
Lymphocytes/µL
391–5610
224–7840
755–9870
1328–7520
414–8100
Eosinophils/µL
45–1761
23–1357
44–1176
59–352
33–528
Monocytes/µL
213–1980
47–1053
38–1780
112–1066
33–1668
Basophils/µL
67–200
0–340
11–540
54–264
44–225
Platelets ×103/µL
0.176–499
120–732
36–784
0.194–296
106–255
Plasma protein (g/dL)
5.7–9.5
ND
ND
ND
ND
Fibrinogen, milligram per deciliter (mg/dL)
200–500
ND
0–700
100–600
200–300
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Suidae and Tayassuidae (Wild Pigs, Peccaries)
Chapter 58