John F. Timoney
Strangles
Strangles is an acute, highly contagious disease characterized by fever, inflammation of the upper respiratory tract mucous membranes, purulent nasal discharge, and abscesses of the mandibular and retropharyngeal lymph nodes. The causative organism, Streptococcus equi subsp equi, is an obligate parasite of equids that rarely infects other hosts. S equi is a clonal derivative of a Streptococcus zooepidemicus ancestor, shows very little antigenic variation, and stimulates a protective immune response that clears the infection from most horses during convalescence. SeM (M-like protein) allelic variants of S equi are listed in the database http://pubmlst.org/szooepidemicus/. Infection is maintained by ongoing transmission to susceptible horses and by intermittent shedding from occasional carrier horses with unilateral or bilateral guttural pouch empyema. Most outbreaks begin after introduction of a horse incubating the disease or a horse that has recently recovered but has not cleared the infection. SeM allele determination may be useful in source tracing. S equi–free status is eventually attained in most herds after a strangles outbreak, so many horse farms, geographic areas, and countries (such as Argentina, Japan, and Ireland) have enjoyed strangles-free status for long periods during the past century.
S equi enters the horse through the mouth or nose and immediately attaches to tonsillar tissues in the oropharynx and nasopharynx. Penetration of the surface occurs within a few hours in a susceptible horse and is followed by multiplication of the organism in the follicular tissues of the tonsil. Formation of extracellular microcolonies is accompanied by massive influxes of neutrophils, some of which escape into the nasopharyngeal secretions through the tonsillar crypts and mucosa. After an incubation period of 3 to 11 days, abrupt onset of fever is followed by swelling of one or more lymph nodes. Pyrexia and other signs of the acute phase response, including hyperfibrinogenemia and neutrophilia, develop in part in response to the effects of all or some of the pyrogenic exotoxins SePE-H, I, L, and M. Other key virulence factors are the antiphagocytic SeM, Se18.9, and IdeE proteins and the hyaluronic acid capsule. S equi, unlike S zooepidemicus I (which does not express proteins with high homology to SeM or Se18.9), is therefore highly resistant to phagocytosis and so is visible in infected lymph nodes as extracellular chains composed of hundreds of organisms. Rupture and drainage of abscesses brings clinical relief and return to normalcy. Shedding of S equi in nasal discharges in most horses ceases 2 to 3 weeks after the onset of clinical signs.
Strangles is most severe in young horses. In older, partially immune horses, the disease may manifest as an afebrile catarrhal form associated with smaller, less painful abscesses. Nevertheless, organisms shed by these horses may be highly virulent for young or more susceptible stock. Some horses with no clinical signs may be infected and shed virulent S equi.
Immunology of Strangles
Immune Response
Infection generates an acquired immune response that is protective in approximately 75% of horses for up to 5 years. Foals of previously infected mares may be immune for 3 to 4 months. Convalescent serum and mucosal antibody responses are targeted at more than 20 surface exposed and secreted proteins of S equi. Antibodies against SeM are opsonizing, antibodies against the pyrogenic exotoxins neutralize pyrogenicity, and mucosal immunoglobulin A (IgA) antibodies specific for tonsil-binding proteins may block adhesion. IgGb is the predominant SeM-specific immunoglobulin isotype found in both acute and convalescent sera. Significant increases in this isotype and in IgGa are detectable within 2 weeks after comingling exposure to an infected horse. IgG(T)-specific antibody appears 1 or 2 weeks later. Concentrations of antibody against SeM and other immunoreactive proteins of S equi peak about 5 weeks after exposure and slowly decline over the following 6 months to levels slightly above those at time of initial infection. Nasal mucosal IgA peaks at about 6 weeks, 1 or 2 weeks after mucosal IgGb, and declines at a rate similar to that of specific serum IgGb.
The protective immune response of the horse is poorly understood. Only a few of the proteins responsible for protective immune responses have been defined. SeM and collagen-, fibronectin-, and albumin-binding proteins, such as CNE, ScLC, EAG, FNE, and SFS in various combinations, have protective efficacy in mice. Antibodies against the superantigenic exotoxins SePe-I, L, and M neutralize pyrogenicity in the horse. However, experimental subcutaneous vaccines composed of proteins with sequences unique to S equi, including SeM, or of adhesion and pilus proteins SzSe, CNE, and T antigen (Se51.9), do not elicit protective responses comparable to those induced by inoculation of live attenuated S equi into the subcutis or during recovery from strangles. Heat-inactivated suspensions of S equi (bacterins) are similarly ineffective. The fact that horses recovered from strangles are observed to clear S equi from their tonsils within an hour of intranasal administration of a challenge inoculum and do not make serum antibody responses against its immunogenic proteins supports the conclusion that tonsillar immunity blocks entry of the organism.
Vaccines
The first reported use of strangles vaccine dates from the late 1700s, when Richard Ford, an English veterinarian, practiced a technique that resembled smallpox vaccination whereby the inside of the lip was abraded and rubbed with lint impregnated with pus from a strangles abscess. The resulting infection spread to local lymph nodes, inducing systemic resistance to natural infection. Much later, French military veterinarians used a serum-vaccine approach whereby inoculation of hyperimmune serum was followed by subcutaneous inoculation of a culture of live S equi. Although these procedures were reputed to elicit a high level of protective immunity, they did not become popular because of the frequent occurrence of abscesses at inoculation sites. Nevertheless, they provided evidence that preparations of live S equi could serve as effective vaccines (Table 41-1).
TABLE 41-1
Strangles Vaccines
| Vaccine | Type | Route of Administration | Schedule | Adverse Reactions |
| Pinnacle IN* | Live attenuated nonencapsulated mutant | Intranasal | 2 doses 2 to 3 wk apart; annual booster | Lymphadenitis, purpura, injection site abscesses |
| Equilis StrepE† | Live attenuated aroA deletion mutant | Submucosal (upper lip) | 2 doses 4 wk apart; booster every 3 mo | Lymphadenitis, local inflammation, injection site abscesses |
| Strepgard‡ | Enzymic extract, adjuvanted (Havlogen) | Intramuscular | 2 doses 2-3 wk apart; annual booster | Local systemic reactions, purpura |
| Strepvax II§ | Acid extract adjuvant (aluminum hydroxide) | Intramuscular | 3 doses at 3-wk intervals; annual booster | Local systemic reactions, purpura |
| Equivax S‖ | Acid extract adjuvant (aluminum hydroxide) | Intramuscular | 3 doses at 3-wk intervals; annual booster | Local systemic reactions, purpura |
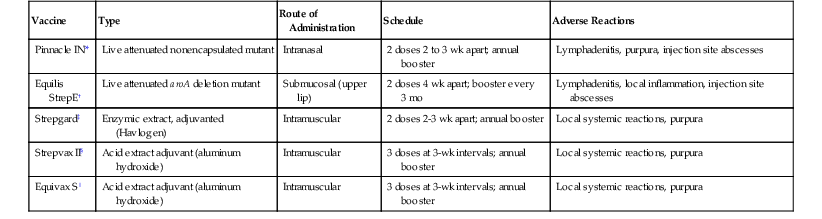
* Zoetis, Kalamazoo, Michigan.
† MSD Animal Health, Hoddesdon, UK.
‡ Intervet, Millsboro, Delaware.
§ Boehringer Ingelheim, St. Joseph, Missouri.
‖ Pfizer Animal Health, NZ, Auckland, New Zealand.
Bacterins
Bacterins produced by exposure of log-phase cultures to gentle heat and introduced in Australia in the 1940s and in North America in the 1960s did not elicit protection comparable to that induced by natural infection. These bacterins often caused local and systemic reactions and were replaced by safer extract vaccines.
Extract Vaccines
Immunogenic proteins of S equi, including SeM extracted by hot acid or mutanolysin plus detergent and adsorbed to aluminum hydroxide, have been widely used as strangles vaccines in North America and have proved highly potent with regard to inducing SeM-specific serum IgGb, but not mucosal IgA, antibody responses. These vaccines are inoculated intramuscularly or subcutaneously and elicit serum antibody responses 7 to 10 days later. Responses are Th2 cytokine-driven, with dominance of SeM-specific serum IgGb and IgG(T). Mucosal IgA or cell-mediated immune responses of value in intracellular killing of S equi in tonsillar follicular tissue are not elicited. Naïve horses and foals require two or three doses at an interval of 2 weeks followed by annual booster doses. Colostral levels of antibody are boosted by prepartum vaccination of mares 1 month before expected date of foaling.
The efficacy of extract vaccines, as for bacterins, has been disappointing and significantly inferior to immunity induced by natural infection. For example, a reduction of 50% in the clinical attack rate has been observed in vaccinates a few weeks after the final booster dose under conditions of heavy exposure. In addition, concerns related to adverse reactions, including muscle stiffness, injection-site abscesses, and purpura hemorrhagica, have reduced acceptance of these vaccines. Prior screening of valuable horses for SeM-specific serum antibody is useful in predicting the risk for purpura. Vaccination is contraindicated when SeM-specific serum antibody titers exceed 1 : 1600 or when the horse has had clinical strangles within the previous 2 years.
There is no persuasive evidence that autogenous bacterins are superior to commercial vaccines. Given the clonality of S equi and the absence of significant variation in its immunogenic proteins, an autogenous vaccine is unlikely to stimulate protective responses not elicited by extracts or earlier commercial bacterins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


