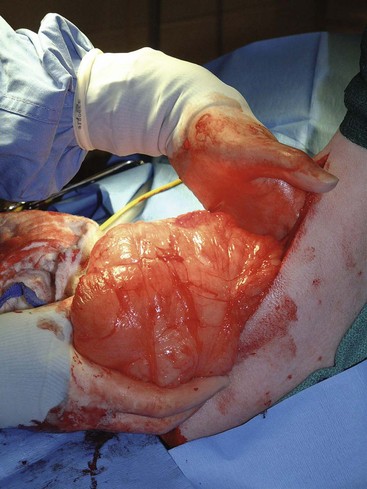
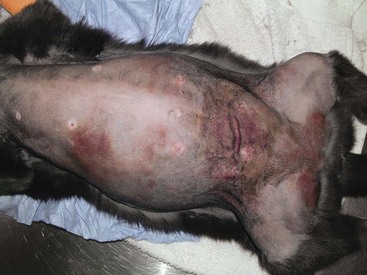
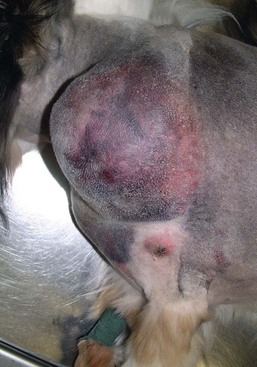
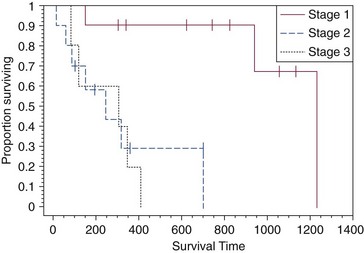
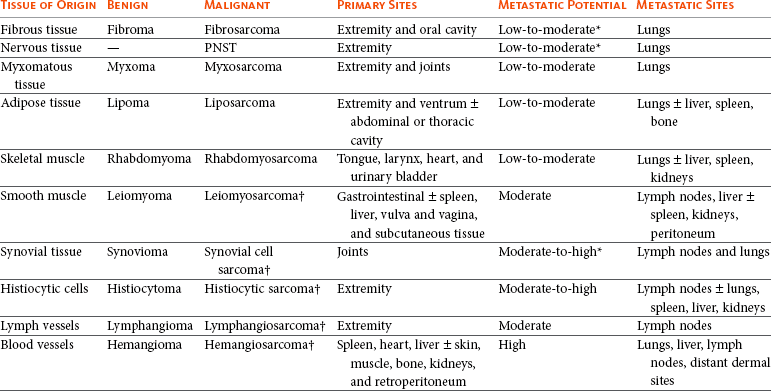
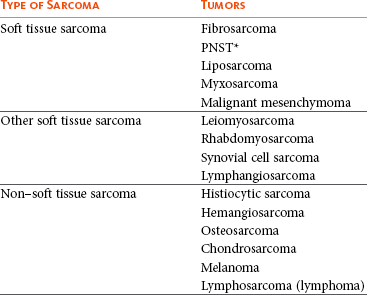
21 Soft tissue sarcomas are a heterogeneous population of mesenchymal tumors that comprise 15% and 7% of all skin and subcutaneous tumors in the dog and cat, respectively.1 The annual incidence of soft tissue sarcomas in companion animals is about 35 per 100,000 dogs at risk and 17 per 100,000 cats at risk.2 In dogs, sarcomas have been associated with radiation, trauma, foreign bodies, orthopedic implants, and the parasite Spirocerca lupi.3–9 Most soft tissue sarcomas are solitary tumors in middle-aged to older dogs and cats. There is no specific breed or sex predilection for soft tissue sarcomas with the possible exception of synovial cell sarcomas in dogs. Earlier reports indicate a slight male predilection10,11; however, in a recent study, males and females were equally represented.12 Another exception is the occurrence of rhabdomyosarcoma in young dogs.13 Soft tissue sarcomas tend to be overrepresented in large breed dogs. Soft tissue sarcomas are a heterogeneous group of tumors whose classification is based on similar pathologic appearance and clinical behavior. Sarcomas arise from mesenchymal tissues and have features similar to the cell type of origin (Table 21-1). These tumors originate in connective tissues, including muscle, adipose, neurovascular, fascial, and fibrous tissue, and can give rise to benign and malignant entities. Only malignant soft tissue sarcomas will be covered in this chapter. Malignant neoplasms in this category include fibrosarcoma, peripheral nerve sheath tumor (PNST; also known as malignant schwannoma, neurofibrosarcoma, or hemangiopericytoma), myxosarcoma, undifferentiated sarcoma, liposarcoma, malignant fibrous histiocytoma, and rhabdomyosarcoma (Table 21-2).14,15 The term soft tissue sarcoma generally excludes those tumors of hematopoietic or lymphoid origin. Hemangiosarcoma (covered briefly here), mast cell sarcoma, oral sarcoma, osteosarcoma, and chondrosarcoma are covered separately in other chapters. Feline sarcomas and vaccine-associated sarcomas are covered in a separate section at the end of this chapter. Table 21-1 Histiogenic Classification and Metastatic Potential of Canine Soft Tissue Sarcomas PNST, Peripheral nerve sheath tumor. *Dependent on histologic grade. †Atypical soft tissue sarcoma with higher metastatic rate of metastasis to regional lymph node. Table 21-2 Tumors listed under “Soft tissue sarcoma” have similar biologic behavior characterized by local aggression and low-to-moderate metastatic potential. Tumors listed under “Other soft tissue sarcoma” are atypical soft tissue sarcomas because of different location (e.g., leiomyosarcoma and synovial cell sarcoma) or higher metastatic rate (leiomyosarcoma, rhabdomyosarcoma, lymphangiosarcoma ± synovial cell sarcoma). Some sarcomas not considered as soft tissue sarcomas are discussed in this chapter because of similarities in location to other soft tissue sarcomas (e.g., histiocytic sarcoma and dermal hemangiosarcoma).133 PNST, Peripheral nerve sheath tumor. *PNSTs include tumors previously classified as hemangiopericytoma, malignant schwannoma, and neurofibrosarcoma. • They tend to appear as pseudoencapsulated soft-to-firm tumors but have poorly defined histologic margins or infiltrate through and along fascial planes, and they are locally invasive. • Local recurrence after conservative surgical excision is common. • Sarcomas tend to metastasize hematogenously in up to 20% of cases. • Regional lymph node metastasis is unusual (except for synovial cell sarcoma).10 • Histopathologic grade is predictive of metastasis, and resected tumor margins predict local recurrence.16 • Measurable or bulky (>5 cm in diameter) tumors generally have a poor response to chemotherapy and radiation therapy (RT). Soft tissue sarcomas present a diagnostic challenge. Many of these tumors have histologic patterns with overlapping features not only among themselves but also with a variety of other neoplasms with different histogenesis. The development of immunocytochemical procedures, the availability of monoclonal antibodies and polyclonal antibodies to various tissue markers, and tissue microarray technology have improved diagnosis of soft tissue sarcomas in human pathology and, to a limited degree, in veterinary pathology.12,17–22 The histologic nomenclature for some sarcomas may vary from pathologist to pathologist. Before the initiation of the appropriate therapy for the treatment of soft tissue sarcomas, it is necessary to know the histologic type, size, site, and grade and the stage of disease. Histologic grading (e.g., low, intermediate, or high or I, II, III) is assigned after histologic characterization from adequate biopsy specimens (Table 21-3). Soft tissue sarcoma is a collective term used to describe a number of different types of tumors with similar histologic features and biologic behavior. The histogenesis of soft tissue sarcomas is controversial and may be difficult to differentiate on the basis of routine histologic and immunohistochemical analysis. Some pathologists have recommended the use of a more generic term such as soft tissue sarcoma or spindle cell tumor of canine soft tissue because of the difficulty in differentiating tumors such as fibrosarcoma, PNST, and hemangiopericytoma.23 Moreover, histologic distinction of tumor type is not clinically important because most soft tissue sarcomas have a similar biologic behavior (i.e., locally aggressive with a low-to-moderate distant metastatic rate). Some types of soft tissue sarcomas covered briefly in this chapter are hemangiosarcomas, lymphangiosarcomas, and synovial cell sarcomas, which are atypical because their biologic behavior is different with a higher rate and different distribution of metastasis. Nodular Fasciitis (Fibromatosis, Pseudosarcomatous Fibromatosis) Nodular fasciitis is a benign nonneoplastic lesion arising from the subcutaneous fascia or superficial portions of the deep fascia in dogs. These lesions are usually nodular, poorly circumscribed, and very invasive.24 Histologically, nodular fasciitis is characterized by large plump or spindle-shaped fibroblasts in a stromal network of variable amounts of collagen and reticular fibers with scattered lymphocytes, plasma cells, and macrophages.24 The morphologic and pathologic characteristics of nodular fasciitis can result in these lesions being misdiagnosed as fibrosarcoma. Infantile desmoid-type fibromatosis is a variant of nodular fasciitis and is characterized by fibroblast proliferation with a dense reticular fiber network and mucoid material.25 Wide excision of both nodular fasciitis and infantile desmoid-type fibromatosis lesions is usually curative.26 Local recurrence is possible with incomplete resection. These tumors do not metastasize.24 Most fibrosarcomas arise from the skin, subcutaneous tissue, or oral cavity and represent malignant fibroblasts. Tumors can be well differentiated, exhibiting spindle-shaped tumor cells with scant cytoplasm. The more anaplastic tumor is very cellular with closely packed spindle-shaped fibroblasts showing many mitotic figures and marked cellular pleomorphism.27 Tumors tend to occur in older dogs and cats with no breed or sex predilection; however, one reference states a higher predilection in golden retrievers and Doberman Pinschers.28 A unique form, histologically low-grade, yet biologically high-grade fibrosarcoma, is seen in the oral cavity and has a tendency to grow quite large and invade deeper structures, including bone. Metastasis can be seen in up to 20% of the cases.19 Metastasis is rare, but these tumors are infiltrative, with microscopic tumor cells invading along fascial planes, and often recur after surgical excision. Peripheral Nerve Sheath Tumor (Neurofibrosarcoma, Malignant Schwannoma, Hemangiopericytoma) The PNSTs are malignant tumors of nerve sheath origin and have been referred to as neurofibrosarcoma, malignant schwannoma, and hemangiopericytoma. The confusion regarding hemangiopericytoma is the fact that blood vessel pericyte origin has yet to be proven in dogs. Most hemangiopericytomas will have features of nerve sheath tumors histologically.27 Malignant PNSTs will stain positive with S-100 and vimentin, indicating peripheral nerve origin.29 Regardless of the nomenclature, these tumors can occur anywhere in the body. A PNST may involve nerves away from the brain or spinal cord (peripheral group), or they may involve nerves immediately adjacent to the brain or spinal cord (root group) or the brachial or lumbosacral plexus (plexus group).30 The true peripheral form is much more treatable than the plexus or root form. Despite appearing encapsulated at surgery, these tumors are similar to fibrosarcomas and occur as poorly defined tumors without histologic encapsulation. Most of the tumors are adherent to deeper tissues and may infiltrate underlying fascia, muscle, and skin. Although these tumors are considered malignant, they have a modest metastatic rate. As with fibrosarcomas, local recurrence for PNSTs is common following conservative surgery. They tend to grow slowly and can range in size from 0.5 cm to over 10 to 12 cm in diameter. In some cases, they can easily be confused with lipomas on initial clinical examination.27 PNSTs located in the proximal axial region may result in the compression of nerves. The vast majority of cases will show signs of unilateral lameness, muscle atrophy, paralysis, and pain.30 They can invade the spinal cord, and about 50% will invade the cord if a grade III tumor is diagnosed.30 However, local disease usually limits survival before metastasis occurs. The reader is referred to Chapter 33, Section F, for a complete discussion of histiocytic disorders because the biology, nomenclature, and management of this collection of benign and malignant conditions continue to evolve. Malignant fibrous histiocytoma as a histopathologic diagnosis, however, is considered to be more consistent with soft tissue sarcomas in general and is managed with guidelines discussed later in this chapter. Lipomas are benign tumors of adipose tissue. Variants of lipomas have been reported and include angiolipoma and angiofibrolipoma.31 Lipomas are relatively common in older dogs, especially in subcutaneous locations, and are rarely symptomatic. Lipomas can also occur in the thoracic cavity, abdominal cavity, spinal canal, and vulva and vagina of dogs and can cause clinical abnormalities secondary to either compression or strangulation.32–40 Parosteal and infiltrative lipomas have been rarely reported and these tumors can have a more aggressive behavior despite their benign histologic appearance.41–46 Marginal resection is recommended for lipomas that interfere with normal function; however, the majority are asymptomatic and do not require surgical intervention. Lipomas can be differentiated from liposarcomas based on morphologic and histologic appearance. Histologically, lipomas have indistinct nuclei and cytoplasm resembling normal fat, whereas liposarcomas are characterized by increased cellularity, distinct nuclei, and abundant cytoplasm with one or more droplets of fat.38 Surgical resection is usually curative, but local recurrence has been reported.39 Intermuscular lipomas are a variant of the subcutaneous lipoma and are located in the intermuscular region of the caudal thigh of dogs, particularly between the semitendinosus and semimembranosus muscles (Figure 21-1).47 Clinically, intermuscular lipomas appear as a slow-growing, firm, and fixed mass in the caudal thigh region and may occasionally cause lameness.47 Cytologic analysis of fine-needle aspirates is usually diagnostic. The recommended treatment is surgical resection, involving blunt dissection and digital extrusion, and placement of either a Penrose or negative-suction drain. Seromas are a common complication in dogs in which a drain is not used. The prognosis is excellent with no recurrence reported in 11 dogs following surgical removal.47 Infiltrative lipomas are uncommon tumors composed of well-differentiated adipose cells without evidence of anaplasia. These tumors cannot be readily distinguished from the more common simple lipoma by cytology or small biopsy specimens. They are considered “benign” and do not metastasize. However, infiltrative lipomas are locally aggressive and commonly invade adjacent muscle, fascia, nerve, myocardium, joint capsule, and even bone.42,48,49 Computed tomography (CT) is used to better delineate these tumors; however, they do not contrast enhance and differentiating infiltrative lipomas from normal fat is problematic.47 One retrospective analysis of 16 cases reported a 4 : 1 female-to-male ratio.44 Aggressive treatment, including amputation, may be necessary for local control. RT can be considered either alone or in combination with surgical excision.46 Liposarcomas are uncommon malignant tumors originating from lipoblasts in older dogs.50 Liposarcomas apparently do not arise from malignant transformation of lipomas. Specific causes are not known, but foreign body–associated liposarcoma has been reported in one dog.5 There is no breed or sex predilection.50 They are commonly reported in subcutaneous locations, especially along the ventrum and extremities, but can also occur in other primary sites such as bone and the abdominal cavity. Liposarcomas are differentiated from lipomas based on morphologic appearance and cytologic characteristics. Liposarcomas are usually firm and poorly circumscribed. They are locally invasive with a low metastatic potential. Metastatic sites include the lungs, liver, spleen, and bone.27,50 The prognosis for liposarcoma is good with appropriate surgical management. The median survival time (MST) following wide surgical excision is 1188 days; this is significantly better than either marginal excision or incisional biopsy, which have MSTs of 649 days and 183 days, respectively (Figure 21-2).50 Liposarcoma is histologically classified as well-differentiated, myxoid, round cell (or poorly differentiated), pleomorphic, or dedifferentiated. This classification scheme has clinical and prognostic importance in humans because pleomorphic liposarcomas have a high metastatic rate, myxoid liposarcomas are more likely to metastasize to extrapulmonary soft tissue structures, and well-differentiated liposarcomas are unlikely to metastasize.51–53 In a retrospective study in dogs, histologic subtype was not prognostic, but metastatic disease was more common in dogs with pleomorphic liposarcomas.50 Figure 21-2 Kaplan-Meier survival curve of 56 dogs with liposarcoma treated with either incisional biopsy, marginal resection, or wide excision. The median survival time (MST) is significantly longer, at 1188 days, following wide surgical resection than less aggressive techniques. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Baez JL, Hendrick MJ, Shofer FS, et al: Liposarcomas in dogs: 56 cases (1989-2000), J Am Vet Med Assoc 224:887, 2004.) J Am Vet Med Assoc Leiomyomas and leiomyosarcomas are tumors arising from smooth muscle cells. The gastrointestinal (GI) tract is most commonly affected, but other primary sites include the spleen, liver, genitourinary tract, retroperitoneal space, vessel wall, and subcutaneous tissue.54–57 Paraneoplastic syndromes associated with smooth muscle tumors, particularly GI leiomyomas and leiomyosarcomas, include hypoglycemia, nephrogenic diabetes insipidus, and secondary erythrocytosis.55,58–61 Leiomyosarcomas are malignant tumors with a moderate metastatic potential, depending on the primary site.55,62 The metastatic rate for dogs with hepatic leiomyosarcoma is apparently 100% but is approximately 50% for other primary intraabdominal sites and 0% for dermal smooth muscle tumors.55,61,63,64 Regional lymph nodes, mesentery, and liver are the most common metastatic sites for GI leiomyosarcoma, although other sites include the spleen, kidneys, and peritoneum.55,61–64 Leiomyosarcoma is the second most common GI tumor in dogs and has a predilection for the jejunum and cecum, but any region of the GI tract can be affected from the esophagus to the rectum.55,61–63,65 An immunohistochemical review of previously diagnosed GI leiomyomas and leiomyosarcomas has resulted in the vast majority of these tumors being reclassified as GI stromal tumors (GISTs) or GI stromal-like tumors.66,67 Leiomyosarcomas have strong immunoreactivity to actin and desmin and rarely stain positively with c-kit, CD34, or S-100 protein. In contrast, true GISTs are consistently associated with mutations of the tyrosine kinase receptor gene c-kit and will have strong immunoreactivity to c-kit and CD34 proteins with variable reactivity to actin, desmin, and S-100 protein.62,65–67 The GI stromal-like tumors have an identical immunohistochemical profile to GISTs, except they do not express c-kit.66 Older dogs are more commonly affected and there is no sex or breed predisposition.55,61–63 In contrast, there is a male predisposition for GI leiomyomas and these tumors have a predilection for the stomach rather than the jejunum and cecum.62,68 Presenting signs can include inappetence, weight loss, vomiting, diarrhea, polyuria, polydipsia, anemia, and hypoglycemia.55,61–63 Surgical resection is the recommended treatment for dogs with leiomyosarcomas. Intestinal perforation with localized to diffuse peritonitis is relatively common in dogs with GI leiomyosarcoma and is reported in up to 50% of cases.61 Local tumor control is good following complete resection, but recurrence has been reported following incomplete resection of a gastric leiomyoma and cutaneous leiomyosarcomas.64,66,68 Prolonged survival and possibly cure has been reported following surgery in dogs with gastrointestinal leiomyosarcoma and GIST tumors.54,55,59,67 Prognostic factors in humans with GIST include tumor size, metastasis, and histologic criteria such as tumor necrosis, number of mitotic figures, and proliferating cell nuclear antigen (PCNA) index69; however, prognostic factors have not been investigated in dogs with leiomyosarcoma. The MST for dogs with GI leiomyosarcoma and GIST surviving the immediate postoperative period is up to 37.4 months, with 1-, 2-, and 3-year survival rates of 75% to 83%, 62% to 67%, and 60%, respectively.55,61,63,66,67 In addition, metastasis did not have a negative impact on survival time in one report with a 21.7 month MST for dogs with documented metastasis at the time of surgery.61 However, other investigators have found metastasis significantly decreases survival time.63 The MST is 8 months for dogs with splenic leiomyosarcoma, and all dogs with hepatic leiomyosarcoma in one series had evidence of metastasis at initial surgery and were euthanized.55 Rhabdomyosarcomas are rare malignant tumors originating from myoblasts or primitive mesenchymal cells capable of differentiating into striated muscle cells.70 In dogs, rhabdomyosarcomas are most frequently reported to arise from skeletal muscle of the tongue, larynx, myocardium, and urinary bladder. They are locally invasive with a low-to-moderate metastatic potential. Metastatic sites include the lungs, liver, spleen, kidneys, and adrenal glands.70 Rhabdomyosarcomas are histologically classified as embryonal, botryoid, alveolar, and pleomorphic. The histologic diagnosis of rhabdomyosarcoma is difficult and immunohistochemical staining for vimentin, skeletal muscle actin, myoglobin, myogenin, and myogenic differentiation (MyoD) may be required for definitive diagnosis.71 Embryonal rhabdomyosarcomas have a predilection for the head and neck region of older dogs, such as the tongue, oral cavity, and larynx. In contrast, botryoid rhabdomyosarcoma commonly arises in the urinary bladder of young, female large breed dogs with Saint Bernard dogs possibly being overrepresented in one data set.70 Botryoid tumors are characterized by their grapelike appearance. The histologic classification scheme for rhabdomyosarcoma has prognostic significance in humans, but this has not been investigated in dogs. In humans, botryoid rhabdomyosarcoma has a good prognosis, embryonal rhabdomyosarcoma has an intermediate prognosis, and alveolar rhabdomyosarcoma has a poor prognosis.70,72 Rhabdomyosarcomas, particularly those involving the extremities, have a high rate of metastasis in humans; major metastatic sites include the lungs, lymph nodes, and bone marrow. The metastatic potential and prognosis in dogs with rhabdomyosarcoma have not been determined because it is rarely diagnosed and even more rarely treated with curative intent. However, disease-free and overall survival times have been encouraging in the few dogs treated with surgical resection with or without RT and chemotherapy.73–77 In humans, in contrast to many other types of soft tissue sarcomas, multimodality treatment with surgery, RT, and chemotherapy has significantly improved survival rates, particularly in children with embryonal and botryoid rhabdomyosarcoma.51,53,72 Lymphangiosarcomas are rare tumors seen in young dogs and cats that arise from lymphatic endothelial cells.27,78,79 They are usually soft, cystic-like, and edematous, usually occurring in the subcutis (Figure 21-3).27 In most cases, clinical signs are associated with extensive edema and drainage of lymph through the skin or a cystic mass. Aspiration may reveal a fluid-filled mass. Histopathologically, these tumors resemble normal endothelial cells and may be confused with hemangiosarcomas because of the vascular channels; however, red blood cells are not seen.27 Lymphangiosarcomas tend to have a moderate-to-high metastatic potential.80 In a single case report, a dog treated with surgery that had local recurrence had a complete response following doxorubicin chemotherapy with no evidence of recurrence or metastasis 9 months after remission.81 Hemangiomas and hemangiosarcomas (HSAs) are tumors of vascular endothelial origin. Hemangioma is a benign tumor that can occur in a variety of sites, including skin, liver, spleen, kidneys, bone, tongue, and heart.82–84 Dermal hemangiomas may be induced by ultraviolet (UV) light in short-haired dogs with poorly pigmented skin.27 Despite their benign biologic behavior, hemangiomas can cause severe anemia secondary to tumor-associated blood loss.82,84 In humans, hemangiomas can spontaneously regress or be responsive to intralesional corticosteroids, but this has not been observed in dogs.27 Complete surgical resection is usually curative. HSAs are highly malignant tumors. The most common primary sites in cats and dogs involve visceral organs, especially the spleen, right atrial appendage, and liver.24 Other primary sites include the skin, pericardium, lung, kidneys, oral cavity, muscle, bone, genitourinary tract, and peritoneum and retroperitoneum.24,85 A thorough review of HSAs is presented in Chapter 33, Section A. The discussion here is limited to dermal and subcutaneous HSAs. Primary dermal HSAs have a predilection for light-haired or poorly pigmented skin on the ventral abdomen and preputial region of dogs, particularly those confined to the dermis, and solar radiation has been proposed as a cause of dermal HSAs in dogs.86 Canine cutaneous HSAs are clinically staged according to depth of involvement with stage I tumors confined to the dermis, stage II tumors extending into subcutaneous tissue, and stage III HSAs involving the underlying muscle (Figure 21-4).86 Stage II and III dermal HSAs are typically large and poorly circumscribed and have a bruised appearance that can be mistaken for a traumatic hematoma. The recommended treatment is wide surgical resection and adjuvant doxorubicin.87 Doxorubicin-based chemotherapy protocols have been investigated in dogs with nonresectable stage II and III cutaneous HSAs, with approximately 40% of dogs showing a measurable response with a median response duration of 53 days.88 Treatment with doxorubicin-based chemotherapy protocols may downstage dogs with large subcutaneous and intramuscular HSAs and thus decrease the size of the tumor, allowing for complete surgical excision.88 Clinical staging provides prognostic information on the success of local treatment, metastatic potential, and survival time. Dogs with stage I dermal HSAs have a complete surgical resection rate of 78%, 30% metastatic rate with all metastases occurring in distant dermal sites, and a MST of 780 days.86 In contrast, dogs with stage II and III hypodermal HSAs have a complete surgical resection rate of only 23%, principally because these tumors are larger and less well circumscribed; are 60% metastatic to lungs, regional lymph nodes, and distant dermal sites; and have a significantly lower MST of 172 to 307 days (Figure 21-5).86 For dogs with aggressive local control (using adequate surgery with or without RT) of subcutaneous or intramuscular HSA, the use of adjuvant doxorubicin results in encouraging survival times. The median disease-free interval (DFI) and survival time for dogs with subcutaneous and intramuscular HSAs treated with surgery and doxorubicin are 1553 and 1189 days and 266 and 273 days, respectively.87 Feline cutaneous HSAs are usually solitary tumors in older cats, with a mean age of 11.5 to 12.5 years and males possibly being overrepresented.89,90 Unlike cutaneous hemangiomas, which have no site predilection, cutaneous HSAs occur primarily in poorly pigmented skin, particularly the skin of the pinna, head, and ventral abdomen and subcutaneous tissue of the inguinal region.89–91 Local tumor control is poor following surgical resection, with local recurrence reported in 50% to 80% of cases at a median of 420 days postoperatively.89–91 Metastasis has been reported but occurs less frequently than in dogs with dermal and hypodermal HSAs.89–91 The MST of greater than 1460 days for cats treated with wide surgical resection is significantly better than the MST of 60 days reported for untreated cats with cutaneous HSAs.90 Synovial cell sarcomas are malignant tumors arising from synoviocytes of the joint capsule and tendon sheath. The two types of synoviocytes are type A synoviocytes, which are phagocytic and resemble macrophages, and type B synoviocytes, which are fibroblastic.92 Synovial cell sarcomas have been classified as monophasic or biphasic, depending on the proportion of malignant epithelial (synovioblastic) and mesenchymal (fibroblastic) cells within the tumor. However, this classification scheme was adopted from human medicine and is probably not applicable in small animals because of the rarity of epithelial cells in canine synovial cell sarcomas.92 To add further confusion to the nomenclature, synovial cell sarcomas and histiocytic sarcomas are often considered to be different types of joint tumors, but both may originate from synovial cells, with synovial cell sarcomas arising from type B synoviocytes and histiocytic sarcomas arising from type A synoviocytes. Expression of CD18 does not differentiate between the macrophages (or type A synoviocytes) and dendritic antigen-presenting cell (APC) lineage of histiocytic cells (P.F. Moore, personal communication). Recent immunohistochemical evidence, however, indicates that periarticular histiocytic sarcomas do arise from subsynovial dendritic APCs and not type A synoviocytes (P.F. Moore, unpublished data). Rare canine synovial myxomas have been reported. They were most common in large breed middle-aged dogs93 and were reported most often at the stifle or a digit and may be confused with the more common synovial cell sarcoma. The diagnosis of synovial cell sarcoma is controversial and immunohistochemistry (IHC) is recommended to differentiate synovial cell sarcomas from other types of joint tumors.12,94,95 The immunohistochemical panel should include vimentin and cytokeratin for synovial cell sarcoma, CD18 for histiocytic sarcoma, and actin for malignant fibrous histiocytoma.94 However, other investigators have questioned the value of IHC in the diagnosis of synovial cell sarcoma.95 Synovial cell sarcomas are histologically graded from I to III based on criteria such as nuclear pleomorphism, mitotic figures, and necrosis, and this grading scheme provides prognostic information.12 Synovial cell sarcomas are locally aggressive with a moderate-to-high metastatic potential, depending on histologic grade.12 Up to 32% of dogs with synovial cell sarcomas have evidence of metastatic disease at diagnosis and 54% by the time of euthanasia.10,12 Regional lymph nodes and lungs are the most common metastatic sites.12 Synovial cell sarcomas have a greater metastatic potential and a higher incidence of lymph node metastasis compared to typical soft tissue sarcomas. Synovial cell sarcomas are rare in cats, but feline and canine synovial cell sarcomas are similar in terms of histologic appearance, biologic behavior, and distribution of metastatic lesions.96 In dogs, synovial cell sarcomas usually occur in large breeds, with a predisposition for flat-coated and golden retrievers. Middle-aged dogs are most commonly affected, and there is no sex predilection. Synovial cell sarcomas usually involve the larger joints, particularly the stifle, elbow, and shoulder, but any joint can be affected. Lameness is the most common presenting complaint and can be confused with other orthopedic conditions.12 Radiographic features of synovial cell sarcomas in dogs include periarticular soft tissue swelling and bone invasion, ranging from an ill-defined periosteal reaction to multifocal punctate osteolytic lesions, involving bones on either side of the joint.12,92 Bone involvement is rare in cats.96 Limb amputation is recommended for treatment of the local tumor because local tumor recurrence is significantly lower compared to marginal or wide resection.12,95 Local tumor recurrence following limb amputation, known as stump recurrence, occurs at a relatively higher rate than other types of tumors for which limb amputation is commonly performed.12 Thus the level of amputation should be as proximal as possible to minimize the risk of local tumor recurrence, such as forequarter amputation for synovial cell sarcomas of the thoracic limb and coxofemoral disarticulation or hemipelvectomy for synovial cell sarcomas of the pelvic limb. The role of adjuvant therapy is unknown, but synovial cell sarcomas in humans are more responsive to chemotherapy agents such as anthracyclines and ifosfamide than many other types of soft tissue sarcomas.51 Doxorubicin-based chemotherapy protocols may be warranted in dogs with nonmetastatic grade III synovial cell sarcomas.12,97 Prognostic factors in dogs with synovial cell sarcoma include clinical stage, histologic grade, and extent of surgical treatment. Dogs with evidence of lymph node or lung metastasis at diagnosis have a MST of less than 6 months compared to 36 months or greater if there is no evidence of metastasis.12 The MST for dogs treated with limb amputation is 850 days, which is significantly better than the 455 days reported following marginal resection.95 Finally, the MST for dogs with grade III synovial cell sarcomas is 7 months and significantly worse than either grade I or II synovial cell sarcomas, with MSTs greater than 48 months and 36 months, respectively.12 In one study comparing canine joint tumors, the metastatic rate and MST for dogs with synovial cell sarcoma was 25% and 31.8 months, 0% and 30.7 months for dogs with synovial myxoma, 91% and 5.3 months for dogs with histiocytic sarcoma, and 100% and 3.5 months for dogs with other types of periarticular sarcomas.94 Myxosarcomas are neoplasms of fibroblast origin with an abundant myxoid matrix composed of mucopolysaccharides. These rare tumors occur in middle-aged or older dogs and cats. The majority are subcutaneous tumors of the trunk or limbs,27 but there are reports of myxosarcomas arising from the heart, eye, and brain.98–100 These tumors tend to be infiltrative growths with ill-defined margins.27 Malignant mesenchymomas are rare soft tissue sarcomas comprising a fibrous component with two or more different varieties of other types of sarcoma.24 Malignant mesenchymomas have been reported in the lungs, thoracic wall, liver, spleen, kidney, digits, and soft tissue.101–106 They have a slow rate of growth and can grow very large. Metastasis has been reported.105,106 The outcome for dogs with splenic mesenchymomas is better than other types of splenic sarcomas, with a MST of 12 months and a 1-year survival rate of 50%.101 Soft tissue sarcomas generally present as a slow-growing mass anywhere in the body. Rapid tumor growth, intratumoral hemorrhage, or necrosis can be seen in some cases. Symptoms are directly related to site of involvement and tumor invasiveness. However, one exception is tumor hypoglycemia, which has been reported in dogs with smooth muscle tumors.59,107 There is marked variability in the physical features of soft tissue sarcoma, but they are generally firm and adherent (fixed) to skin, muscle, or bone. Often, soft tissue sarcomas can be soft and lobulated, mimicking lipomas. Intraabdominal tumors often compress the GI tract and patients may present with vomiting, diarrhea, and melena. A mass may be palpable, and weight loss and anorexia is common. Leiomyosarcomas are seen most commonly in the GI tract, spleen, and urogenital tract. GI leiomyosarcomas generally result in intestinal obstruction and may cause intestinal perforation leading to peritonitis.61 Rhabdomyosarcomas, seen in young dogs and involving the bladder, present with signs of hematuria, dysuria, and rarely, hypertrophic osteopathy.108,109 Fine-needle aspiration (FNA) is recommended to exclude other differentials (e.g., abscesses, cysts, or mast cell tumors [MCTs]). However, cytologic evaluation of FNA may not be sufficient for definitive diagnosis because false-negative results can occur because of nonrepresentative samples subject to variable degrees of necrosis and poor exfoliation of cells in comparison to epithelial and round cell tumors.24 In one study in which FNA was performed on soft tissue sarcomas from 40 dogs, 15% of dogs were incorrectly diagnosed and a further 23% were nondiagnostic.110 Biopsy methods for definitive preoperative diagnosis of soft tissue sarcomas include needle-core, punch, incisional, or excisional biopsies. The biopsy should be planned and positioned so that the biopsy tract can be included in the curative-intent treatment, whether it be surgery with or without RT, without increasing the surgical dose or size of the radiation field. Excisional biopsies are not recommended because they are rarely curative and the subsequent surgery required to achieve complete histologic margins is often more aggressive than surgery following core or incisional biopsies, resulting in additional morbidity and treatment costs. Furthermore, multiple attempts at resection, including excisional biopsy, prior to definitive therapy have a negative impact on survival time in dogs with soft tissue sarcomas.111 Diagnostic tests performed for work-up and clinical staging depend on the type of soft tissue sarcoma, especially with atypical forms (e.g., HSAs, histiocytic sarcomas, lymphangiosarcomas, and synovial cell sarcomas), but usually involve routine hematologic and serum biochemical blood tests, three-view thoracic radiographs, and regional imaging of the soft tissue sarcoma. Blood tests are usually within the normal reference range for most dogs with soft tissue sarcomas; however, there are some notable exceptions. Hematologic abnormalities such as anemia and thrombocytopenia are relatively common in dogs with disseminated histiocytic sarcomas and HSAs.24,112,113 Hypoglycemia has been reported in dogs with intraabdominal leiomyomas and leiomyosarcomas.55,58,59,107 Excessive production of insulin-like growth factor II has been implicated as the cause of hypoglycemia in humans with mesenchymal tumors and this has also been demonstrated in one dog with a gastric leiomyoma.114–116 Imaging studies of the local tumor may be required for planning of the surgical approach or RT if the tumor is fixed to underlying structures or located in an area that may make definitive treatment difficult, such as the pelvic region. Three-dimensional (3D) imaging techniques such as CT and magnetic resonance imaging (MRI) are particularly useful for staging local disease.117 Other imaging modalities for staging of the local tumor include survey radiographs, ultrasonography, angiography, and nuclear scintigraphy. Diagnostic tests for staging of metastatic disease include thoracic radiographs, abdominal ultrasonography or advanced imaging, and FNA or biopsy of the regional lymph nodes. Three-view thoracic radiographs should be performed prior to definitive treatment because the lungs are the most common metastatic site for typical soft tissue sarcomas.16 Lymph node metastasis is uncommon. FNA or biopsy of regional lymph nodes should be performed in dogs with clinically abnormal lymph nodes, high-grade (III) soft tissue sarcomas, or atypical soft tissue sarcomas having a high rate of metastasis to regional lymph nodes, such as HSAs, histiocytic sarcomas, lymphangiosarcomas, synovial cell sarcomas, leiomyosarcomas, and possibly rhabdomyosarcomas.* Abdominal imaging is recommended for the assessment of metastasis to intraabdominal organs, especially the lymph nodes, spleen, and liver, in animals with suspected intraabdominal neoplasia (e.g., GI leiomyosarcoma or splenic sarcoma) or advanced stage and high-grade pelvic limb soft tissue sarcoma. A modified staging system has been described for soft tissue sarcomas in dogs.24 The American Joint Committee on Cancer (AJCC) staging system currently used in humans with soft tissue sarcomas has been substantially modified from the original staging system, on which the modified animal staging system is based. The most important change to the AJCC staging system is categorization of local disease, with less emphasis on tumor size, which is an arbitrary assignment, and greater emphasis on depth of invasion.51,120 A superficial tumor is defined as a soft tissue sarcoma located above the superficial fascia and that does not invade the fascia, whereas a deep tumor is located deep to the superficial fascia, invades the fascia, or both.108 Based on the current AJCC staging system, an updated modified staging system for animals is summarized in Box 21-1. The stage grouping takes into account both clinical staging criteria (TNM staging system) and histologic grade. The original and updated staging systems for animals with soft tissue sarcomas have not been investigated and the prognostic significance of either of these systems is unknown. There are over 20 different histologic subtypes of soft tissue sarcomas described in dogs, but the vast majority of these subtypes have a very similar biologic behavior characterized by locally aggressive invasiveness and a low-to-moderate metastatic potential depending on histologic grade.16 Local tumor control is the most important consideration in the management of soft tissue sarcomas because of their locally aggressive behavior. As such, surgical resection is the principal treatment for dogs with soft tissue sarcomas. RT also plays an important role in local tumor control, especially for incompletely resected and unresectable soft tissue sarcomas. However, definitive treatment options depend on histologic subtype (especially for atypical soft tissue sarcomas such as HSAs, histiocytic sarcomas, and lymphangiosarcomas), tumor location, clinical stage, histologic grade, and completeness of surgical margins.24,121 A strategy for managing dogs with typical soft tissue sarcomas is presented in Figure 21-6. Soft tissue sarcomas are locally aggressive tumors that grow along paths of least resistance and invade surrounding tissue, resulting in the formation of a pseudocapsule of compressed viable tumor cells.24 The pseudocapsule gives the false impression of a well-encapsulated tumor. However, surgical removal of the encapsulated mass without adequate margins will result in incomplete resection and a high risk of local tumor recurrence.16 The minimum recommended margins for surgical resection are 3 cm lateral to the tumor and one fascial layer deep to the tumor (Figure 21-7).121,122 Biopsy tracts and any areas of fixation, including bone and fascia, should be resected en bloc with the tumor using the recommended surgical margins. Radical surgery such as limb amputation or hemipelvectomy may be required to achieve adequate surgical margins and local tumor control. Alternatively, planned multimodal therapy with marginal surgical excision and postoperative RT may be limb sparing and reduce patient morbidity. The resected tumor should be pinned out to the original dimensions to prevent shrinkage during formalin fixation (Figure 21-8)123; the lateral and deep margins should be inked to aid in histologic identification of surgical margins; and any areas of concern should be tagged with suture material, inked in a different color, or submitted separately for specific histologic assessment. Surgical margins and tumor grade are important in determining the need and type of further treatment. For instance, tumor grade is important in deciding whether a soft tissue sarcoma resected with complete but close surgical margins (i.e., 1 to 3 mm) will require further local treatment because surgical margins may be adequate with a low-grade soft tissue sarcoma but not with a high-grade soft tissue sarcoma.121,122
Soft Tissue Sarcomas
Incidence and Risk Factors
Pathology and Natural History*
Specific Tumor Types
Tumors of Fibrous Origin
Fibrosarcoma
Tumors of the Peripheral Nerves
Histiocytic Disorders
Tumors of Adipose Tissue
Intermuscular Lipoma
Infiltrative Lipoma
Liposarcoma

Tumors of Smooth Muscle
Tumors of Skeletal Muscle
Tumors of Vascular and Lymphatic Tissue
Hemangioma
Hemangiosarcoma
Tumors of Synovial Tissue
Tumors of Uncertain Histogenesis
Malignant Mesenchymoma
History and Clinical Signs
Diagnostic Techniques and Work-Up
Clinical Staging
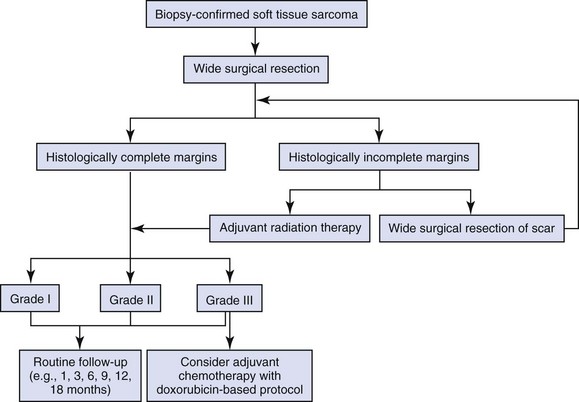
Treatment
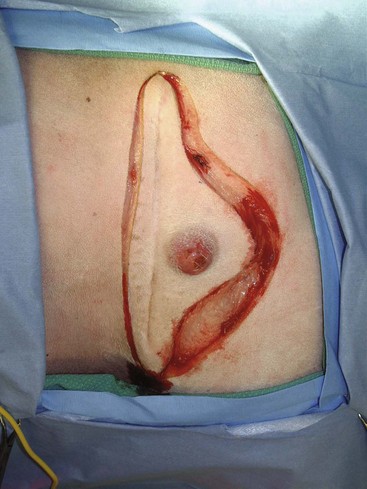
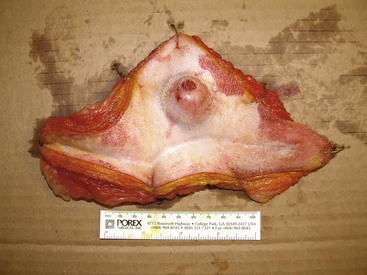
Surgery
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Soft Tissue Sarcomas