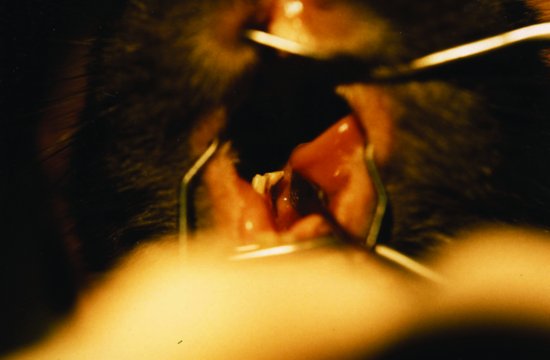
Figure 4.2 A chinchilla showing cheek tooth elongation leading to mouth ulcers and anoirexia.
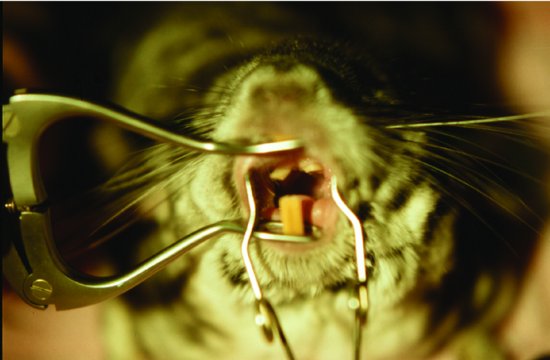
Figure 4.3 A guinea pig showing cheek tooth elongation and buccal spurs leading to ulceration of the tongue and anorexia.
Second, the fibre is essential to stimulate gut motility. These species are hind gut fermenters and rely on the microflora of the hind gut to digest food by breaking down the cellulose. Fibre is converted by the intestinal microflora into volatile fatty acids, which decrease the pH of the caecum and large bowel, so preventing bacterial overgrowth and minimising enteritis problems. Without sufficient fibre, the hind gut fermenting species develop a mucoid enteropathy, with intermittent constipation, diarrhoea and colic.
Rabbits may also be inclined to overgroom themselves, consume large volumes of fur as a ‘fibre source’ and so develop fur balls (trichobezoars) which may completely block the stomach. Rabbits have a requirement for 12–16% crude fibre, which is similar to guinea pigs, but chinchilla dietary fibre levels have been quoted as varying from 15% to 35% (Hoefer, 1994). The high levels for chinchillas are explained by their natural diet, which is chiefly composed of poor-quality, highly abrasive and fibrous grasses in the arid and high altitude Andes Mountains of South America. The lack of such fibre in pet diets may well account for the chinchilla’s appalling susceptibility to molar malocclusion problems. It is recommended that chinchillas and rabbits are fed good quality hay, dried grass or dried grass pellets as 30–50% of their diet as a minimum. The sole feeding of pelleted and dry mix foods currently available leads to a major fibre shortage which leads to lack of dental wear, gastrointestinal upsets and obesity.
Unlike rabbits and other species, guinea pigs do not eat until their calorific requirements are satisfied and then stop, but instead eat until their digestive system is full, irrespective of how diluted the energy of the diet is by increased fibre. Recommended minimum crude fibre levels for guinea pigs are 10%, with an average of 13% being routinely offered (Hillyer et al., 1997).
Vitamins
Fat-soluble vitamins
Vitamin A
In small herbivores the diet frequently contains only the vitamin A precursors, as carotenoid plant pigments, whereas in ferrets, vitamin A itself is obtained from animal proteins and does not need to be synthesised. In small herbivores, the most important form of these precursors in terms of how much vitamin A can be produced from it is beta-carotene. Because it is fat-soluble, vitamin A can be stored in the body, primarily in the liver.
Hypovitaminosis A is an uncommonly seen problem in small mammals. This is because green vegetables are good providers of the beta-carotenes. A problem can arise with rodents that develop an addiction to seeds such as sunflower seeds and peanuts. Hypovitaminosis A in rabbits can cause infertility, foetal resorption, abortion, stillbirth and neurological defects such as hydrocephalus. In ferrets it can cause infertility, poor coat and fluid retention (anasarca). Recommendations for dietary levels include 7000 IU/kg for rabbits (Carpenter & Kolmstetter, 2000). The low levels may be met by as little as 200 g of carrot per day for a 2 kg rabbit, but would require 70 kg of oats to provide the same levels!
Hypervitaminosis A rarely occurs naturally, but may be induced by overdosing with vitamin A injections at 1000 times or more the daily recommended doses. If this occurs, acute toxicity develops with mucus membrane and skin sloughing, liver damage and frequently, death within 24–48 hours. It may also be seen in ferrets fed predominantly the livers of prey items. In these cases excess bony production may occur similar to the syndrome seen in cats.
Vitamin D
This vitamin is primarily concerned with calcium metabolism. Cholecalciferol is manufactured in the small mammal’s skin, a process enhanced by ultraviolet light. Small mammals kept indoors therefore produce much less of this compound, and this can lead to deficiency. Cholecalciferol must then be activated in the liver and kidneys before it can function in calcium metabolism. Once formed into its active metabolite it acts in concert with parathyroid hormone to increase reabsorption of calcium at the expense of phosphorus from the kidneys, to increase absorption of calcium from the intestines and to mobilise calcium from the bones, all of these functions increasing the blood’s calcium levels.
Hypovitaminosis D3 causes problems with calcium metabolism and leads to rickets. This is exacerbated by low-calcium-containing diets, a typical sufferer being an indoor kept small mammal, fed an all seed diet in the case of a rodent or an all meat and no calcium supplement diet for a ferret. This leads to well-muscled, heavy rodents or ferrets, with poorly mineralised bones, flaring of the epiphyseal plates at the ends of the long bones and concomitant bowing of the limbs, especially the tibiotarsal bones. Recommended minimum levels are 3.5–9 IU/g of feed for rodents and lagomorphs (Wallach & Hoff, 1982) and 0.5–1.5 IU/g dry matter for sugar gliders.
Hypervitaminosis D3 occurs due to over supplementation with D3 and calcium and leads to calcification of soft tissues, such as the medial arterial walls, and the kidneys, creating hypertension and causing organ failure. Recommended maximum levels are 2000 IU/kg dry matter of food fed for most small mammals (Wallach & Hoff, 1982).
Vitamin E
This compound is found in several active forms in plants, the most active being alpha-tocopherol. It is used as an antioxidant and in immune system function.
Hypovitaminosis E in ferrets results in a yellow discolouration in body fat deposits, haemolytic anaemia and a progressive paresis of the limbs. In addition, a series of firm swellings underneath the skin in the inguinal area may be seen. In rabbits a similar disease is seen with hindlimb paresis and white muscle degeneration. In hamsters, deficiencies have been reported as causing muscular weakness, ocular secretions and death, often due to cardiac muscle damage. Hypovitaminosis E may occur due to a reduction in fat metabolism or absorption as can occur in small intestinal, pancreatic or biliary diseases, or due to a lack of green plant material, the chief source of the compound, in the diet. Recommended levels for rabbits are 40 mg/kg dry matter of food offered (National Research Council, 1978).
In addition, feeding diets high in unsaturated fats (such as oily fish) may use up the body’s reserves of vitamin E and so induce signs of hypovitaminosis E. This has occurred in ferrets and mink fed such a diet.
Hypervitaminosis E is extremely rare.
Vitamin K
Because of its production by bacteria, it is very difficult to get a true deficiency of vitamin K, although absorption will again be reduced when fat digestion or absorption is reduced, as in, for example, biliary or pancreatic disease.
Ferrets eating prey which has been killed by warfarin will show signs of deficiency. Rabbits and guinea pigs eating large amounts of sweet clovers can also experience a relative deficiency. The signs of disease that are seen are due to increased internal and external haemorrhage, but vitamin K also has some function in calcium/phosphorous metabolism in the bones and this may also be affected.
Water-soluble vitamins
Vitamin B1 (thiamine)
Hypovitaminosis B1 is uncommon, but may be seen in ferrets fed raw saltwater fish which contain thiaminases. When a relative deficiency occurs, neurological signs such as opisthotonus, weakness and head tremors are seen. Ferrets can suffer from a condition known as Chastek syndrome. The symptoms may include salivation, paralysis, incoordination, pupillary dilation and easily induced convulsions, which are characterised by strong ventral flexion of the neck. Evidence of dilated cardiomyopathies is seen at post-mortem. The recommended minimum level for most small mammals is around 6–7 mg/kg of diet dry matter (Wallach & Hoff, 1982). Sugar gliders fed primarily on pollen/nectar replacers may develop thiamine deficiencies owing to the low levels of thiamine in these food sources. Clinically this can present with seizures. Ensuring adequate mineral vitamin pre-loading of insects in the sugar glider diet will prevent this.
Vitamin B2 (riboflavin)
Hypovitaminosis B2 is very rare and produces growth retardation, roughened coat, alopecia, excess scurf and cataract formation.
Niacin
Rodents fed a high proportion of one type of seed such as sweetcorn can become deficient in niacin. They exhibit blackening of the tongue (known as pellagra) and oral mucosa, retarded growth, poor coat quality and scaly dermatitis. Rats may also have anaemia and porphyrin-encrusted noses.
Biotin
Deficiency may occur in animals fed large amounts of unfertilised, raw eggs, the whites of which contain the antibiotin vitamin, avidin. This can be seen in ferrets and chipmunks when greater than 10% of the diet is composed of raw egg. Deficiency produces exfoliative dermatitis, toes may become gangrenous and slough off and ataxia may be observed. The recommended minimum requirement is 0.12–0.34 ppm food as dry matter for small mammals (Wallach & Hoff, 1982).
Folic acid
Deficiency occurs mainly because of the folic acid inhibitors that are present in some foods such as cabbage and other brassicas, oranges, beans and peas. The use of trimethoprim sulphonamide drugs also reduces gut bacterial folic acid production. Deficiency causes a number of problems, such as failure in reproductive tract maturation, macrocytic anaemia due to failure of red blood cell maturation and immune system cellular dysfunction.
Choline
Because of their interactions, the need for choline is dependent on levels of folic acid and vitamin B12. That is, a deficiency may be caused if the latter are deficient, as may occur in an animal fed a diet high in fats. Deficiency causes retarded growth, disrupted fat metabolism and fatty liver damage. Recommended minimum requirements for small mammals are 880–1540 mg/kg food as dry matter.
Vitamin C
There is no direct need for this vitamin in small mammals other than the guinea pig, as vitamin C may be synthesised from glucose in the liver. In some marsupials it can be synthesised in the kidney. In the sugar glider it is not currently known whether it can synthesise vitamin C, but the Virginia opossum can do so in its liver. The guinea pig lacks the enzyme l-gluconolactone oxidase and therefore cannot convert glucose to ascorbic acid, so has a dietary requirement for vitamin C. However, during disease processes, particularly those conditions that affect liver function, it may be beneficial to the recovery process in all species to provide a dietary source of vitamin C.
Vitamin C is required for the formation of elastic fibres and connective tissues and is an excellent antioxidant, similar to vitamin E. Deficiency leads to ‘scurvy’. Signs of scurvy include poor wound healing, increased bleeding due to capillary-wall fragility, gingivitis and bone alterations. These include the swelling of long bones close to joints (the epiphyseal plates), which become very painful. In addition, crusting occurs at mucocutaneous junctions such as the eyes, mouth and nose, as well as loosening of the teeth due to periodontal ligament weakening.
Vitamin C also helps in the absorption of some minerals, such as iron, from the gut.
The daily recommended requirement for guinea pigs is 10 mg/kg body weight for maintenance, increasing to 30 mg/kg body weight during gestation, although treatment of scurvy recommends levels of 50–100 mg/kg until resolution of clinical signs (Harkness & Wagner, 1995). This may be given by injection, or soluble human vitamin C tablets may be placed in the drinking water each day if the guinea pig is still drinking adequately. In addition, fresh fruit and vegetables as well as a specific supplemented guinea pig diet, should always be fed. Many deficiencies occur due to guinea pigs being housed with rabbits and therefore fed only dry rabbit food which has no supplemental vitamin C.
Minerals
Macro-minerals
Calcium
Calcium has a wide range of functions, the two most obvious being its role in the formation of the skeleton and mineralisation of bone matrix, and its use in muscular contraction. The active form of calcium in the body is the ionic double-charged molecule Ca2+. Low levels of this form, even though the overall body reserves of calcium may be normal, lead to hyperexcitability, fitting and death.
Calcium levels in the body are controlled by vitamin D3, parathyroid hormone and calcitonin.
The ratio of calcium to phosphorus is very important. As one increases the other decreases and vice versa. A ratio therefore of 2:1 calcium to phosphorus is desirable in juvenile and lactating small mammals and 1.5:1 for adults. Excessive dietary calcium (>1%) though reduces the use of proteins, fats, phosphorus, manganese, zinc, iron and iodine. In rabbits, this is exacerbated by their unique method of calcium control, in that they have no ability to reduce calcium absorption from the gut, as do other species. Instead, all available calcium is absorbed from the diet, and any excess must then be excreted through the kidneys. This leads to excess calcium excretion into the urine, the formation of calcium carbonate crystals and urolithiasis. In addition, as rabbits are herbivorous, their urine pH is alkaline, and as calcium carbonate (limestone) is less soluble in alkaline environments it precipitates more readily in the urine, forming crystals. Levels of calcium >4% dry matter for rabbits will lead to soft tissue mineralisation in sites such as the aorta and kidneys. Calcium deficiency and metabolic bone disease problems are common in omnivorous marsupials such as the sugar glider which consume sap and insects in large quantities – both of which are deficient in calcium. Hypocalcaemic tetany has also been reported in sugar gliders.
Phosphorus
Like calcium, phosphorus is used in bone formation, but it is also used in cell structure and energy storage. It is widespread in plant and animal tissues, but in the former it may be bound up in unavailable form as phytates. Levels of phosphorus are controlled in the body as for calcium, the two being in equal and opposite equilibrium with each other. Therefore, if dietary phosphorus levels exceed calcium levels appreciably (a maximum of twice the calcium levels on average) the parathyroid glands become stimulated to produce more parathyroid hormone in an effort to restore the balance. This causes nutritional secondary hyperparathyroidism which leads to progressive bone demineralisation and then renal damage due to the high circulating levels of parathyroid hormone. High dietary phosphorus also reduces the amount of calcium which can be absorbed from the gut, as it complexes with the calcium present there. This can be a big problem for ferrets that are fed pure meat diets with no calcium or bone supplement, and in rodents that are predominantly seed eaters, as cereals are high phosphorus/low calcium foods. Feeding green vegetables or supplementation with calcium powders may therefore be necessary. In the case of ferrets a standard ferret complete diet, or whole rodent prey, should be fed to avoid this.
Potassium
As with larger mammals, this is the major intracellular positive ion. Rarely is there a dietary deficiency. Severe stress can cause hypokalaemia due to increased kidney excretion of potassium due to elevated plasma proteins, as can persistent diarrhoea. Hypokalaemia can lead to cardiac dysrhythmias, muscle spasticity and neurological dysfunction. Other symptoms are stunted growth, ascites, abnormally short hair and reduced appetite. Potassium is present in high amounts in certain fruits, such as bananas. It is controlled in equilibrium with sodium by the adrenal hormone aldosterone which promotes sodium retention and potassium excretion.
Sodium
This is the main extracellular positive ion and regulates the body’s acid–base balance and osmotic potential. In conjunction with potassium, it is responsible for nerve signals and impulses. Rarely does a true dietary deficiency occur, but hyponatraemia may occur due to chronic diarrhoea or renal disease. This disrupts the osmotic potential gradient in the kidneys and water is lost leading to further dehydration. Excessive levels of sodium in the diet (>10 times recommended) lead to poor coat, polyuria, hypertension, oedema and death.
Chlorine
This is the major extracellular negative ion and responsible for maintaining acid–base balances in conjunction with sodium and potassium. Deficiencies are rare, but if they do occur, retarded growth and kidney disease are commonly seen.
Micro-minerals (trace elements)
Copper
Copper is used in haemoglobin synthesis, collagen synthesis and in the maintenance of the nervous system. Copper toxicosis has been reported in ferrets in the United States as a possible hereditary storage disease. The symptoms are of liver disease, as seen in Bedlington Terriers (Brown, 1997). Deficiency has been reported in hamsters as a cause of poor coat quality and generalised alopecia. Minimum recommended levels are 13–20 ppm (Wallach & Hoff, 1982).
Iodine
Iodine’s sole function is in thyroid hormone synthesis, which affects metabolic rate. Deficiency causes goitre, and has knock-on effects on growth causing stunting, stillbirths and neurological problems. It is a relatively uncommon finding in small mammals but may occur in species such as rabbits that are fed large volumes of goitrogenic (iodine inhibiting) plants such as cabbage, kale and Brussels sprouts.
Iron
This is essential, as with larger mammals, for the formation of the oxygen-carrying part of the haemoglobin molecule. Absorption from the gut is normally relatively poor, as the body is very good at recycling its own iron levels. Vitamin C enhances iron uptake from the gut. In sugar gliders Dierenfield et al. (2006) recommend less than 50 μg/g of dry diet of iron to avoid iron storage disease, which can damage the liver.
Manganese
Deficiency has been reported as causing poor bone growth, with limb shortening as a consequence. Recommended daily requirements are from 40 to 120.7 ppm with the higher dosages for guinea pigs (Wallach & Hoff, 1982).
Selenium
The main role of selenium is as part of the antioxidant enzyme glutathione peroxidase, with which vitamin E is also involved. Its functions are therefore similar to those of vitamin E in that it helps keep peroxidases from attacking polyunsaturated fats in cell membranes. A general deficiency in both selenium and vitamin E will lead to liver necrosis, steatitis and muscular dystrophy. The selenium content of plants is dependent on where they were grown and the levels of selenium in the soil. Recommended minimum requirements are still not clearly defined for small mammals in general.
Zinc
This is a vital trace element for wound healing and tissue formation, forming part of a number of enzymes. Deficiencies can occur in young, rapidly growing guinea pigs and chinchillas fed on plant material high in phytates such as cabbage, wheat bran and beans. In addition, high dietary calcium decreases zinc uptake. Deficiency produces retarded growth and poor skin quality with increased scurf and hyperirritability. Zinc deficiency alopecia is particularly seen at about day 50 of gestation in chinchillas with hair regrowth occurring 2–3 weeks after parturition (Smith et al., 1977). Minimum recommended requirements are 20–122 ppm for small mammals (Wallach & Hoff, 1982). Zinc toxicosis has been reported in ferrets, with anaemia, lethargy and hindlimb paresis, and was associated with feeding from zinc galvanised buckets (Donnelly, 1997).
Requirements for young and lactating small mammals
Rabbit
Neonatal rabbits nurse for only 3–5 minutes at a time once or twice in a 24-hour period and are totally dependent on their mother’s milk up to day 21 postpartum (Okerman, 1994). At this time they should be weighed, as solid foods offered will be increasingly consumed, and weight losses may be seen if they do not eat enough of this. The doe may be offered increasingly more pelleted dry foods before weaning, as her energy demands increase to 3.5 times maintenance by peak lactation. Ad-lib dry food is therefore often advocated for the doe at this stage. It should be noted though that levels of food for the doe should not start to be dramatically increased until 5–7 days after parturition. Early overfeeding can cause excessive milk production, and mastitis will result if the kits do not have enough appetite to empty the mammary glands at each sitting. For hand-rearing formulas, see page 28.
Growing kits require higher levels of vitamin D3 and calcium than their adult counterparts. To ensure that this is received, a balanced diet should be offered, combining pelleted food, good quality grass hay and some greens. Dry foods should be carefully chosen. Many are balanced nutritionally, but only if the rabbit consumes all parts equally. Rabbits are concentrate selectors (that is they will preferentially pick out those foods containing the highest calories in their environment and eat them first). Therefore, if offered one of the ‘muesli’-type diets, it will eat all of the fatty, carbohydrate foods first, and, if provided ad-lib, they will not get around to eating the high fibre, calcium-containing grass pellets. Hence, it is advised either to use a homogenous pelleted diet where all of the pellets are exactly the same, or to feed enough in 24 hours so that the bowl is completely emptied before offering more. Access to unfiltered natural sunlight is also advised, even if for only 15–20 min/day, to ensure sufficient vitamin D3 synthesis. Lactating does will also have higher calcium requirements, and so their consumption of pelleted diet and grass products (both high in calcium) will increase. Care should be taken, though, not to overdo pelleted diets at the expense of good quality hay/grass, as excessive amounts of calcium and vitamin D3 can come to be present, leading to reno- and urolithiasis as well as soft tissue mineralisation.
Ferret
Young ferrets have a higher calorific requirement than adults, as do lactating jills, needing 1.5–2 times maintenance adult calorie levels. The protein requirement is a minimum of 35% in young growing ferrets and lactating jills with a fat level of a minimum of 25% as dry matter (Kupersmith, 1998). For rearing formulas
see page 35.
Rodents, chinchilla and guinea pig
The guinea pig sow, when lactating, has a requirement for vitamin C which increases from 10 to 30 mg/kg per day. She also has the usual increases in calcium, energy and protein demands. The demands for increased calories are particularly important, as the long gestation of the guinea pig (average 63 days) and the frequent litter size of 3–4 piglets, place huge stresses on the sow. If these increased requirements are not met, then a condition known as pregnancy toxaemia, or ketosis ensues. This is when a lack of available calories leads to increased fat mobilisation. If this is combined with a glucose deficit the fats are converted into chemicals known as ketones. These produce metabolic acidosis in addition to the hypoglycaemic state. Death can follow within 24 hours. Prevention is geared towards avoiding obesity and sudden dietary change, both of which set up the condition.
Young guinea pigs are frequently not hungry for the first 12–24 hours after birth because they have brown fat reserves. They should not be force-fed during this time. Young chinchillas and guinea pigs eat solid foods practically from day 1 after parturition. It is important therefore to ensure high-fibre foods are offered preferentially at this stage so as to avoid them developing into fussy eaters in later life.
Rodents such as rats and gerbils have been quoted as needing a dietary protein level of 20–26% during and prior to pregnancy and lactation, as opposed to their more usual 16–18%.
For rearing formulas see page 29.
Omnivorous marsupials
Energy requirements increase significantly in sugar gliders from around 46 kJ/day as basal requirements to 229 kJ/day for growth and activity. Lactation has much higher demands on the female marsupial than gestation, due to the altricial nature of the joeys when born. Many insectivorous marsupials can enter a state of torpor when food sources (mainly the insect source which provides most of the protein, fats and calories) are scarce to conserve energy. Their body temperature will drop and they will appear to be unrousable. It is not uncommon for these periods to last up to 11 hours.
For rearing formulas for sugar gliders and Virginia opossums see page 33.
Requirements for debilitated small mammals
In general, requirements for debilitated animals will vary from 1.5 to 3 times maintenance levels, with the lower levels being for mildly injured or infected animals and the upper levels for burns victims and cases of serious organ damage or septicaemia.
Fluid therapy as additional support is essential, particularly for the herbivorous mammals, which have very high maintenance requirements when compared with cats and dogs (on average 80–100 mL/kg per day). Also, herbivore gut contents are voluminous and need to be kept fluid. For further details see chapters 6 and 8.
Rabbit
The debilitated rabbit may be supported with nasogastric or oral syringe feeding of vegetable-based baby foods (lactose free varieties), or, for preference, with a gruel composed of ground dry rabbit pellets and water as this will supply a better fibre level for gut stimulation. Amounts suggested to feed at any one sitting vary from 3 to 15 mL four to six times daily.
A nasogastric tube (more correctly a naso-oesophageal tube as the tubing must not allow reflux of acid stomach contents into the oesophagus) is placed after first spraying the nose with lignocaine spray. A 3–4 French tube is pre-measured from the extended nose to the seventh rib or caudal end of the sternum and then inserted. Sterile water should be flushed through the tube before and after feeding to ensure it is correctly placed and does not become blocked. The tube may then be glued, taped or sutured to the dorsal aspect of the head and a bung inserted when not in use. It may be necessary to put an Elizabethan collar on the rabbit to prevent removal.
The use of cisapride at a dose of 0.5 mg/kg orally every 8–24 hours (Smith & Bergmann, 1997) is to be advocated in rabbits to stimulate large bowel activity, and encourage the return of normal appetite.
Older rabbits often do better on lower protein (14%) and higher fibre (18%) diets as these reduce the risk of obesity, kidney and liver damage.
Ferret
The debilitated ferret may be supported with nasogastric or oral syringe feeding of meat-based baby foods, or commercially prepared liquid meat-based formulas designed for cats and dogs such as Reanimyl® and Hill’s a/d. Amounts suggested for feeding at any one sitting vary from 2 to 10 mL three to six times daily. It is vitally important that no debilitated ferret goes longer than 4 hours without nutritional support, as they will become rapidly hypoglycaemic.
A feline 3 French nasogastric tube can easily be placed via the nostril after first spraying the area with lignocaine spray. The tube should be premeasured to extend from the nose tip to the level of the seventh rib (i.e. it is really a naso-oesophageal tube, to avoid the acid contents of the stomach refluxing and causing an oesophagitis) and cut to this length. It may then be secured with tissue glue, or taped or sutured to the skin over the forehead. It is advised to fit an Elizabethan collar to prevent the ferret removing the tube. The tube should be flushed with sterile water before and after feeding to ensure the tubing is in the correct position and does not block.
Rodents, chinchilla and guinea pig
Syringe feeding orally or using a straight avian crop tube to administer liquid food directly into the oesophagus are advised. The latter may be stressful, as the rodent must be scruffed prior to administration. Volumes suggested vary from 0.5 mL for a mouse, up to 2.5 mL for a rat at any one sitting, the dose to be repeated 6–8 times daily to ensure correct calorie administration. Diets such as dry rodent pellets ground in a coffee grinder and then added to water to form gruel, or the use of vegetable-based, lactose-free baby foods may be used. Guinea pigs and chinchillas benefit from the use of oral cisapride to stimulate gut motility, as well as the use of gruels made from higher-fibre chinchilla or rabbit pellets. Naso-oesophageal tubes can be attempted for the larger individuals of these two species.
Marsupials
For sugar gliders a typical diet would include the following (Johnson-Delaney, 2010): 15 mL of Leadbeater’s mix (which is in itself made of 150 mL warm water plus 150 mL honey plus one shelled, hard-boiled egg plus 15 g of baby cereal (rice-based) plus 5 g of powdered avian vitamin and mineral supplement); 15 g of an insectivore/carnivore diet; and then various additions as treats such as chopped fruit and live gut fed insects, which should not exceed 10% of the diet. The practice of gut-loading insects with vitamin/mineral supplements is useful and should be familiar to anyone keeping reptiles.
Probiotics
The use of oral probiotics is recommended for small herbivores, to encourage normal digestive function by providing enzymes and to encourage normal pH conditions. Probiotics are best added to the drinking water but may be added to the syringed food to ensure consumption.
References
Bell, J. (1993) Ferret nutrition and diseases associated with inadequate nutrition. Proceedings of the North American Veterinary Conference, Orlando, FL, pp. 719–720.
Brown, S.A. (1997) Basic anatomy, physiology and husbandry. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery (eds E.V. Hillyer & K.E. Quesenberry), pp. 3–13. W.B. Saunders, Philadelphia, PA.
Carpenter, J.W. and Kolmstetter, C.M. (2000) Feeding small exotic mammals. Hill’s Nutrition, pp. 943–960. Mark Mervis Institute, Marceline, MO.
Dierenfield, E.S., Thomas, D. and Ives, R. (2006) Comparison of commonly used diets on intake, digestion, growth and health in captive sugar gliders (Petaurus breviceps). Journal of Exotic Pet Medicine, 15(3), 218–224.
Donnelly, T.M. (1997) Basic anatomy, physiology and husbandry. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery (eds E.V. Hillyer & K.E. Quesenberry), pp. 147–159. W.B. Saunders, Philadelphia, PA.
Harkness, J.E. and Wagner, J.E. (1995) The Biology and Medicine of Rabbits and Rodents, 4th edn, p. 230. Lea & Febiger, Philadelphia, PA.
Hillyer, E.V., Quesenberry, K.E. and Donnelly, T.M. (1997) Biology, husbandry and clinical techniques. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery (eds E.V. Hillyer & K.E. Quesenberry), pp. 243–259. W.B. Saunders, Philadelphia, PA.
Hoefer, H.L. (1994) Chinchillas. Veterinary Clinics North American Small Animal Practice, 24, 103–111.
Hume, I. (1999) Metabolic rates and nutrient requirements. Marsupial Nutrition (ed. I. Hume), pp. 1–34. Cambridge University Press, Cambridge.
Johnson-Delaney, C. (2010) Marsupials. Manual of Exotic Pets (eds A. Meredith & C. Johnson-Delaney), 5th edn, pp. 103–126. BSAVA, Quedgeley, UK.
Kupersmith, D.S. (1998) A practical overview of small mammal nutrition. Seminars in Avian and Exotic Pet Medicine, 7(3), 141–147.
National Research Council (1978) Nutrient Requirements of Laboratory Animals. National Academy Press, Washington, DC.
Okerman, L. (1994) Diseases of Domestic Rabbits, 1st edn. Blackwell Science, Oxford.
Smith, D.A. and Bergmann, P.M. (1997) Formulary. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery (eds E.V. Hillyer & K.E. Quesenberry), pp. 392–403. W.B. Saunders, Philadelphia, PA.
Smith, J.C., Brown, E.D. and Cassidy, W.A. (1977) Zinc and vitamin A: interrelationships of zinc metabolism. Current Aspects in Health and Disease. Alan R. Liss, Inc., New York.
Wallach, J.D. and Hoff, G.L. (1982) Nutritional diseases of mammals. Noninfectious Diseases of Wildlife (eds G.L. Hoff & J.W. Davis), pp. 133–135 and 143–144. Iowa State University Press, Iowa.
Further reading
Booth, R. (2003) Sugar gliders. Seminars in Avian and Exotic Pet Medicine, 12(4), 228–231.
Fraser, M.A. and Girling, S.J. (2009) Rabbit Medicine and Surgery for Veterinary Nurses. Blackwell-Wiley, Oxford.
Harkness, J.E. and Wagner, J.E. (2000) Biology and Medicine of Rabbits and Rodents, 5th edn. Lea & Febiger, Philadelphia, PA.
Keeble, E. and Meredith, A. (2009) Manual of Rodents and Ferrets. BSAVA, Quedgeley, UK.
MacPherson, C. (1997) Sugar Gliders. A Complete Owner’s Manual. Barron’s Educational Series, Hauppage, NY.
Meredith, A. and Flecknall, P. (2006) Manual of Rabbits, 2nd edn. BSAVA, Quedgeley, UK.
Meredith, A. and Johnson-Delaney, C. (2010) Manual of Exotic Pets, 5th edn. BSAVA, Quedgeley, UK.
Okerman, L. (1998) Diseases of Domestic Rabbits, 2nd edn. Blackwell Science, Oxford.
Quesenberry, K.E. and Carpenter, J. (2003) Ferrets, Rabbits and Rodents, 2nd edn. W.B. Saunders, Philadelphia, PA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


