When transferred from one room to another the rabbit must be held close to the handler’s chest. Non-fractious individuals may also be supported with their heads pushed into the crook of one arm, with that forearm supporting the length of the rabbit’s body. The other hand is then used to place pressure on or grasp the scruff region (see Figure 3.2).
Once caught the rabbit may be calmed further by wrapping it in a towel, so that just the head and ears protrude. There are also specific rabbit papooses that encircle the rabbit, but leave the head and ears free. This allows ear blood sampling and oral examinations, but controls their powerful hindlimbs. It is important not to allow them to overheat in this position, as rabbits do not have significant sweat glands and do not actively pant. They can therefore overheat quickly with fatal results if their environmental temperature exceeds 23–25°C.
Rat and mouse
Mice will frequently bite an unfamiliar handler, especially in strange surroundings. First, grasp the tail near to the base and then position the mouse on a non-slip surface. While still grasping the tail, the scruff may now be grasped firmly between thumb and forefinger of the other hand.
Rats will rarely bite unless roughly handled. They are best picked up by encircling the pectoral girdle immediately behind the front limbs with the thumb and fingers of one hand, while bringing the other hand underneath the rear limbs to support the rat’s weight (Figure 3.3). The more fractious rat may be temporarily restrained by grasping the base of the tail before scruffing it with thumb and forefinger.
Under no circumstances should mice or rats be restrained by the tips of their tails as de-gloving injuries to the skin covering them will occur.
Gerbil and hamster
Hamsters can be difficult to handle. If the hamster is relatively tame, cupping the hands underneath the animal is sufficient to transfer it from one cage to another.
Some breeds of hamster are more aggressive than others, with the Russian hamster (also known as the Djungarian or hairy-footed hamster) being notorious for its short temper. In these cases, the hamster should be placed onto a firm flat surface, and gentle but firm pressure placed onto the scruff region with finger and thumb of one hand. As much of the scruff should then be grasped as possible, with the pull in a cranial direction, to ensure that the skin is not drawn tight around the eyes (hamsters have a tendency to proptose their eyes if roughly scruffed) (Figure 3.4). If a very aggressive animal is encountered, the use of a small glass or perspex container with a lid for examination and transport purposes is useful.
Figure 3.4 Method for restraining a hamster. Note the large amount of loose skin which must be grasped and the high position at which it is grasped immediately behind the ears to avoid eye proptosis.

Gerbils are relatively docile, but can jump extremely well when frightened and may bite. For simple transport they may be moved from one place to another by cupping the hands underneath them. For more rigorous restraint, the gerbil may be grasped by the scruff between thumb and forefinger of one hand after placing it onto a flat level surface. It is vitally important not to grasp a gerbil by the tail. The skin will strip off, leaving denuded coccygeal vertebrae, and the skin will never regrow. Jerds and jerboas are related species and handling techniques are the same.
Guinea pig and chinchilla
Guinea pigs are rarely aggressive, but they are highly stressed when separated from their companions and normal surroundings. Dimming the lighting, and reducing noise and other stress can aid in control.
The guinea pig should be grasped behind the forelimbs from the dorsal aspect with one hand, while the other is placed beneath the hindlimbs to support its weight. This is particularly important as the guinea pig has a large abdomen but slender bones and spine. Without supporting the rear end, spinal damage is risked.
Chinchillas are equally timorous and rarely if ever bite. They too can be easily stressed and reducing noise and dimming room lighting can be useful. When restrained they must not be scruffed under any circumstances as this will result in the loss of fur at the site held. This fur-slip will leave a bare patch which will take many weeks to regrow. Chinchillas may actually lose some fur due to stress of restraint, even if no physical gripping of the skin occurs. Some chinchillas, when particularly stressed, will rear up on their hind legs and urinate at the handler with surprising accuracy! It is therefore essential to pick up the chinchilla calmly and quickly with minimal restraint, placing one hand around the pectoral girdle from the dorsal aspect just behind the front legs, and the other hand cupping the hind legs and supporting the chinchilla’s weight. Degus may be handled in a similar fashion, although they are less prone to fur-slip.
Chipmunk
Chipmunks are highly strung, and the avoidance of stress and fear aggression is essential to avoid fatalities. They are difficult to handle without being bitten, unless hand-reared, when they may be scruffed quickly, or cupped in both hands. To catch them in their aviary-style enclosures the easiest method is to use a fine-meshed aviary or butterfly net, preferably made of a dark material. The chipmunk may then be safely netted and quickly transferred to a towel for manual restraint, examination, or injection or induction of chemical restraint.
Marsupials
Sugar gliders are very docile and may be restrained with minimal force. If they need to be held firmly for any reason, one hand can hold the base of the tail and the other the dorsal aspect of the neck. For Virginia opossums that are tame, one had may be used to grasp around the pectoral girdle and the other to support the rear end in a similar fashion to that described for domestic rats. For more aggressive animals leather gardening gloves/gauntlets should be used. They may also be wrapped in towels in a fashion similar to rabbits.
Ferret
Ferrets can make excellent house pets and many are friendly and hand-tame. However, in the United Kingdom, ferrets are most frequently kept for rabbit hunting; hence, many ferrets are not regularly handled and so may be aggressive.
For excitable or aggressive animals, a firm grasp of the scruff, high up at the back of the neck is advised. The ferret may then be suspended while stabilising the lower body with the other hand around the pelvis. In tamer animals, they may be suspended with one hand behind the front legs, cupped between thumb and fingers from the dorsal aspect, with the other hand supporting the hindlimbs. This hold may be varied somewhat in the more lively individuals by placing the thumb of one hand underneath the chin, so pushing the jaw upwards, and the rest of the fingers grasping the other side of the neck. The other hand is then brought under the hindlimbs as support.
Aspects of chemical restraint
Chemical restraint may be necessary for a number of reasons in small mammals:
- Sample collection, such as blood testing or urine collection
- For procedures such as radiography
- Oral examinations
Is the patient fit enough for chemical restraint?
It is important to assess whether the patient is fit enough for a chemical restraint before it is attempted. Factors that should be considered prior to the anaesthesia of small mammals are outlined below.
Low-grade respiratory infections
Many rodents and lagomorphs suffer from low-grade respiratory infection all of their lives. Most will cope with this, but when anaesthetised the respiratory rate slows and respiratory secretions, already thickened or increased due to chronic infection, become more tenacious. This can cause physical blockage of the airways.
Respiratory system anatomy
The majority of the species considered are nose breathers, with their soft palates permanently locked around the epiglottis. Therefore, if the patient has a blocked nose, whether it be due to pus, blood, tumours or abscesses, then respiratory arrest is made much more likely under anaesthetic.
Hypothermia
Because of their small size and resultant high body surface area to volume ratios, these small mammals are prone to hypothermia during anaesthesia. This is due to the cooling effect of the inhaled gases and reduced muscular activity. It is dangerous, therefore, to anaesthetise a patient that is already hypothermic without first treating this condition.
Dehydration
Respiratory fluid losses during drying gaseous anaesthetic procedures are much greater than in cats, dogs or larger species. Hence, putting a severely dehydrated small mammalian patient through an anaesthetic without prior fluid therapy is not advisable.
Pre-anaesthetic management
Weight measurement
It is vitally important to weigh the patient accurately. A mistake of just 10 g in a hamster, say, will lead to an under- or overdosage of 10%! The use of scales that will read accurately down to 1 g in weight is therefore essential.
Blood testing
Blood testing is starting to become much more common in small mammals and should be considered in every clinically unwell or senior patient where a sufficiently large sample may be obtained. Sites for venipuncture are detailed below.
Rabbit
A 25–27 gauge needle may be used in the lateral ear vein. Prior to sampling, apply a local anaesthetic cream to the site and warm the ear. Alternatively, the cephalic or the jugular veins may be used. The latter should be used with caution as it is the only source of blood drainage from the eyes, and so, if a thrombus forms in this vessel, ocular oedema and permanent damage or even loss of the eye may occur.
Rat and mouse
The lateral tail veins may be used. These run on either side of the coccygeal vertebrae and are best seen when the tail is warmed as for the lateral ear vein in lagomorphs. A 25–27 gauge needle is required.
Ferret
The jugular vein is the easiest to access, but may be difficult in a fractious animal. One handler holds onto both front limbs with one hand, clamping the body with forearm and elbow, the other hand placed under the chin and raising the head. Towel restraint may also be utilised to papoose the ferret. Cephalic veins may also be used. Needles of 23–25 gauge suffice.
Guinea pig and chinchilla
The jugular veins are the most accessible. One handler holds both front limbs with one hand and brings the patient to the edge of the table, raising the head with the other hand. The other operator may then take a jugular sample with a 23–25 gauge needle. Lateral saphenous veins may be used in guinea pigs, but chinchillas rarely have any other peripheral vessel large enough to sample.
Gerbil and hamster
These are difficult. With care (particularly in gerbils) the lateral tail veins may be used. Frequently cardiac puncture under anaesthetic is often necessary to obtain a sufficient sample.
Chipmunk
Jugular blood samples may be taken, but in nearly every case anaesthesia is required first.
Marsupials
The lateral or ventral tail vein, cephalic or lateral saphenous may be used. Anaesthesia may be required first though due to the small size of some and potential aggression of others.
Fasting
Rabbit
These do not need to be fasted prior to anaesthesia, as they have a very tight cardiac sphincter preventing vomiting. Starving may actually be deleterious to the patient’s health as it causes a cessation in gut contractility and subsequent ileus. It is important, though, to ensure that no food is present in the mouth at the time of induction, hence a period of 30–60 minutes starvation should be ensured.
Rat and mouse
Because of their high metabolic rate and likelihood of hypoglycaemia, rats and mice need only be starved a matter of 40–60 minutes (mice) to 45–90 minutes (rats) prior to anaesthetic induction.
Guinea pig and chinchilla
These may be starved for 3–6 hours prior to surgery to ensure a relatively empty stomach and reduce pressure on the diaphragm. Again, prolonged starvation (>4 hours) will lead to hypoglycaemia and gut stasis.
Gerbil and hamster
As for Muridae, a period of 45–90 minutes is usually sufficient. Any longer than 2 hours and post-operative hypoglycaemia is a real problem.
Chipmunk
Periods of fasting of 2 hours have been reported as safe.
Marsupial
Smaller marsupials may be starved for 1 hour with larger ones requiring 3–6 hours.
Ferret
These may be starved for 2–4 hours. Many have insulinomas and will develop hypoglycaemia. Also mustelids have a high metabolic rate and short gut transit times.
Pre-anaesthetic medications
Pre-anaesthetic drugs are used because they provide a smooth induction and recovery from anaesthesia, or because they ensure a reduction in airway secretions, act as a respiratory stimulant or prevent serious bradycardia.
Antimuscarinics
Atropine is used in some species such as guinea pigs and chinchillas where oral secretions are high and intubation is difficult. Doses of 0.05 mg/kg have been used subcutaneously 30 minutes before induction. Atropine also acts to prevent excessive bradycardia, which often occurs during the induction phase.
It is not useful in lagomorphs, as around 60% of rabbits have a serum atropinesterase that breaks down atropine before it has a chance to work. Glycopyrrolate, which functions in a similar manner, may be used instead at doses of 0.01 mg/kg subcutaneously.
Tranquilisers
Tranquilisers are used to reduce the stress of induction. Many species will breath-hold during gaseous induction, to the point where they may become cyanotic. In rabbits, the ‘shock’ organ is the lungs, and during intense stress the pulmonary circulation can go into spasm, making the hypoxia due to breath-holding even worse, even to the point of collapse and cardiac arrest!
Acepromazine
Acepromazine (ACP) can be used at doses of 0.2 mg/kg in ferrets, 0.5 mg/kg in rabbits and 0.5–1 mg/kg in rats, mice, hamsters, chinchillas and guinea pigs. In general, it is a very safe premedicant even in debilitated animals. However, it is advised that it not be used in gerbils, as ACP reduces the seizure threshold, and many gerbils suffer from hereditary epilepsy. This author uses a dose of 0.2 mg/kg ACP in combination with 0.05 mg/kg atropine as a premedication for chinchillas.
Diazepam
Diazepam is useful as a premedicant in some species. In rodents, doses of 3 mg/kg can be used, even in gerbils. In rabbits, the benefits are somewhat outweighed by the larger volumes required. In addition, as the drug is oil based, the intramuscular route is employed and this may be painful.
Neuroleptanalgesics
The fentanyl/fluanisone combination (Hypnorm®) may be used at varying doses as a premedicant, a sedative or as part of an injectable full anaesthesia. As a premedicant, doses of 0.1 mL/kg for rabbits, 0.08 mL/kg for rats, 0.2 mL/kg for guinea pigs (one-fifth the recommended sedation doses) can produce sufficient sedation to prevent breath holding and allow gaseous induction. These doses are given intramuscularly 15–20 minutes before induction. However, Hypnorm® is an irritant and large doses in one site may cause post-operative lameness. It can be reversed after the operation with butorphanol at 0.2 mg/kg or buprenorphine at 0.05 mg/kg intravenously. Should a vein not be available, both may also be given intramuscularly.
Fluid therapy
Fluid therapy is a vitally important pre-anaesthetic consideration and will be mentioned below and elsewhere in the book.
Induction of anaesthesia
Injectable agents
Table 3.1 outlines some of the advantages and disadvantages of injectable anaesthetics in small mammals.
Table 3.1 Advantages and disadvantages of injectable anaesthetics.
| Advantages | Disadvantages |
| Easily administered | Delay in reversal |
| Minimal stress | Hypoxia and hypotension common |
| Prevent breath-holding | Tissue necrosis |
| Inexpensive | Organ metabolism required |
Propofol
It may be used in ferrets at 10 mg/kg after the use of a premedicant such as ACP. However, in rabbits and hystricomorphs, apnoea can be a problem, and because it must be given intravenously it is difficult to use in the smaller rodents. Doses of 5–10 mg/kg have been used intravenously in rabbits.
Alfaxalone
Alfaxalone has also been used at 5 mg/kg intravenously in rabbits, but it must be given slowly as apnoea can result.
Ketamine
Ketamine is a dissociative anaesthetic commonly used in small mammals.
Ferret
Ketamine may be used alone for chemical restraint in the ferret at doses of 10–20 mg/kg but, as with cats and dogs, the muscle relaxation is poor and salivation can be a problem. More often, ketamine is combined with other drugs such as the alpha-2 agonists xylazine and medetomidine. In ferrets, 10–30 mg/kg ketamine may be used with 1–2 mg/kg xylazine (Flecknell, 1998), preferably giving the xylazine 5–10 minutes before the ketamine.
Rabbit
Ketamine is used at a dose of 5–10 mg/kg in conjunction with medetomidine at 0.1–0.25 mg/kg and butorphanol at 0.5 mg/kg or with xylazine at 5 mg/kg (Flecknell, 2000). The advantages are a quick and stress-free anaesthetic, but the combination will cause blueing of the mucous membranes due to peripheral shut-down, making detection of hypoxia difficult. Respiratory depression may become a problem and intubation is often advised. Medetomidine may be reversed using atipamezole at 1 mg/kg.
Rat, mouse, gerbil and hamster
Ketamine can be used at 90 mg/kg in combination with xylazine at 5 mg/kg intramuscularly or intraperitoneally in rats and 100 mg/kg ketamine with 5 mg/kg xylazine in hamsters (Harkness & Wagner, 1989). Mice require 100 mg/kg of ketamine and 10 mg/kg xylazine (Orr, 2001). These combinations provide 30 minutes or so of anaesthesia.
In gerbils, the dose of xylazine may be reduced to 2–3 mg/kg as they appear more sensitive to the hypovolaemic effects of the alpha-2 drugs, with ketamine doses at 50 mg/kg (Flecknell, 1998).
Ketamine may also be used at 75 mg/kg in combination with medetomidine at 0.5 mg/kg in gerbils (Keeble, 2001) and rats (Orr, 2001). Mice may require as much as 1 mg/kg medetomidine (Orr, 2001). The advantages of the alpha-2 agonists are that they produce good analgesia (which ketamine does not) and that they may be quickly reversed with atipamezole at 1 mg/kg. Their disadvantages include severe hypotensive effects, and that once administered they are more difficult to control than a gaseous anaesthetic. Alpha-2 agonists also increase diuresis and may exacerbate renal dysfunction.
Guinea pig and chinchilla
Ketamine at 40 mg/kg in conjunction with xylazine at 5 mg/kg can be used in guinea pigs to produce a light plane of anaesthesia (Mason, 1997). Ketamine at 40 mg/kg may also be used with medetomidine at 0.5 mg/kg for guinea pigs (Flecknell, 2001), or ketamine at 30 mg/kg with medetomidine at 0.3 mg/kg for chinchillas (Mason, 1997). These doses may be reversed with 1 mg/kg atipamezole. The response to both of these combinations may be improved after an ACP premedication of 0.25 mg/kg. Alternatively, for chinchillas, a ketamine (40 mg/kg) and ACP (0.5 mg/kg) combination can be used (Morgan et al., 1981). Induction takes 5–10 minutes and typically lasts for 45–60 minutes, but recovery may take 2–5 hours for the non-reversible ACP combination, hence reducing the dose of this drug and using the reversible alpha-2 antagonists may be beneficial. This author uses a combination of 0.2 mg/kg ACP plus 0.05 mg/kg atropine as a premedication followed by 2–4 mg/kg ketamine and 0.02–0.04 mg/kg medetomidine as induction in chinchillas which is suitable for radiography, dental examinations/molar burring and minor invasive procedures.
Fentanyl/fluanisone (Hypnorm® Vetapharma Ltd)
This drug combination is a neuroleptanalgesic licensed for use in rats, mice, rabbits and guinea pigs. Fentanyl is a morphine-/opioid derivative, and fluanisone is the neuroleptic. It is mentioned here particularly because it is specifically licensed for use in rabbits, rats and mice and guinea pigs in the United Kingdom.
Rabbit
Sedation at doses of 0.5 mL/kg intramuscularly (see data sheets) produces sedation and immobilisation for 30–60 minutes, but the analgesic effect from the opioid derivative fentanyl will persist for some time longer. It may however be reversed with 0.5 mg/kg butorphanol intravenously, or 0.05 mg/kg buprenorphine, both of which will counteract the fentanyl and its analgesia and substitute their own pain relief.
Alternatively, to provide anaesthetic depth fentanyl/fluanisone may be combined with diazepam at a dose of 0.3 mL Hypnorm® to 2 mg/kg diazepam given intraperitoneally or intravenously (but in separate syringes as they do not mix). It may also be combined with midazolam (0.3 mL Hypnorm® to 2 mg/kg midazolam) and given intramuscularly or intraperitoneally in the same syringe. Hypnorm® may also be given intramuscularly first and then followed 15 minutes later by midazolam given intravenously into the lateral ear vein. These two combinations provide good analgesia and muscle relaxation with duration of anaesthesia of 20–40 minutes.
The fentanyl part may be reversed with buprenorphine or butorphanol given intravenously. In emergencies, naloxone at 0.1 mg/kg intramuscularly or intravenously may be given, but this provides no substitute analgesia.
Fentanyl/fluanisone combinations are well tolerated in most rabbits, but they can produce respiratory depression and hypoxia.
Rat and mouse
Hypnorm® may be used as sedation only on its own at a dose of 0.01 mL/30 g body weight in mice and 0.4 mL/kg in rats. This produces sedation and immobilisation for 30–60 minutes and may be reversed with buprenorphine or butorphanol as above.
Alternatively, it may be combined with diazepam (mice 0.01 mL/30 g Hypnorm® with 5 mg/kg diazepam intraperitoneally; rats 0.3 mL/kg Hypnorm® with 2.5 mg/kg diazepam intraperitoneally). In this case the diazepam and Hypnorm® are given in separate syringes as they do not mix. Midazolam is miscible with Hypnorm® and for rodents the recommendation is that each drug is individually mixed with an equal volume of sterile water first. These solutions are then mixed together in equal volumes. Of this stock solution, mice receive 10 mL/kg and rats receive 2.7 mL/kg as a single intraperitoneal injection. These two combinations provide anaesthesia for a period of 20–40 minutes.
Guinea pig and chinchilla
Hypnorm® may be used for sedation only on its own at a dose of 1 mL/kg intramuscularly. This may be problematic in guinea pigs as large volumes are required. Hypnorm® is an irritant and may cause lameness when the whole dose is placed in one spot – multiple sites are therefore preferred. It should be noted that it is not licensed for use in the chinchilla in the United Kingdom.
Alternatively, it may be combined with diazepam (1 mL/kg Hypnorm® and 2.5 mg/kg diazepam) in separate syringes and given intraperitoneally, or with midazolam by making the stock solution as described above for rats and mice, and then administering 8 mL/kg of this solution intraperitoneally. Hypnorm® may be reversed with the partial opioid agonists buprenorphine and butorphanol, or with the full antagonist naloxone.
Gaseous agents
Table 3.2 gives some advantages and disadvantages of gaseous anaesthetics in small mammals.
Table 3.2 Advantages and disadvantages of gaseous anaesthetics.
| Advantages | Disadvantages |
| Faster alteration of depth of anaesthesia possible | Increased drying effect on respiratory membranes |
| Recovery times shorter | Hypothermic effect from drying |
| Less organ metabolism | Difficulty in some species which breath-hold |
| Delivered in 100% oxygen so better oxygenation than injectables on their own | May be considerably more expensive |
Isoflurane
Usually a premedication is used as due to its mildly irritant effects on mucus membranes, breath-holding is common, particularly in rabbits. Its advantage over previous gases such as halothane is in its safety for the debilitated patient. Only <0.3% of the gas is metabolised hepatically, the rest merely being exhaled for recovery to occur. Recovery is therefore rapid.
Induction levels vary at 2.5–4% and maintenance usually is 1.5–2.5% assuming adequate analgesia. Breath-holding can still be a problem, even with premedication, but the practice of supplying 100% oxygen to the patient for 2 minutes prior to anaesthetic administration helps minimise hypoxia. Isoflurane is then gradually introduced, first 0.5% for 2 minutes, then, assuming regular breathing, increased to 1% for 2 minutes and so on until anaesthetic levels are reached. This allows a smooth induction.
Sevoflurane
This gas anaesthetic is used commonly in small mammals now, and has an advantage over isoflurane in that breath-holding, when it is used as an induction agent is much reduced, particularly in rabbits. At levels exceeding 4% on induction it can still induce breath-holding, so it is preferable to use it at 4% for induction and 2–3% for maintenance. In guinea pigs, profuse lacrimation as well as salivation occurs with both isoflurane and sevoflurane and therefore it is advisable to use atropine or glycopyrrolate as a premedicant.
Maintenance of anaesthesia
Intubation
As with all gaseous anaesthetics, the placement of an endotracheal tube for maintenance after induction is to be recommended whenever possible. This is relatively straightforward in ferrets, being much the same procedure as for cats.
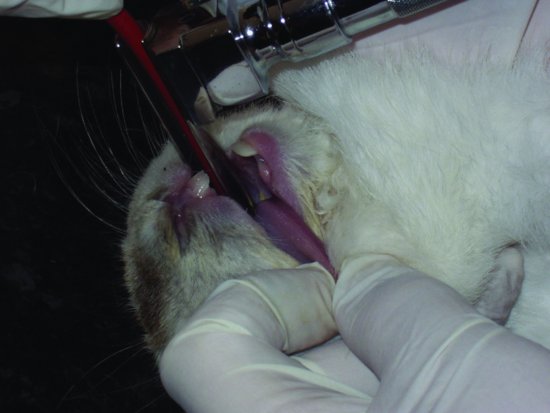
In rabbits, the use of a number 1 Wisconsin flat-bladed paediatric laryngoscope and a 2–3 mm tube is advised. The rabbit is first induced either with an injectable anaesthetic or with an inhalational one. It is then placed in dorsal recumbency, allowing the larynx to fall dorsally and into view. The tongue should be pulled out to one side and the laryngoscope and endotracheal tube are inserted (see Figure 3.5). A guide wire may first be passed through the laryngeal opening, and, once in, the endotracheal tube is threaded over the top and the wire withdrawn.
Figure 3.5 Insertion of a laryngoscope and ET tube in an already anaesthetised rabbit in dorsal recumbency. Note the careful lateral displacement of the tongue to avoid laceration on the incisors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




