CHAPTER 15 Structural and physiologic features of skeletal muscle determine much of its response to injury. Although muscle cells are frequently called muscle fibers or myofibers, they are in fact multinucleated cells of considerable length, which in some animals may approach 1 m. Myonuclei are located peripherally in the cylindrical myofiber (Fig. 15-1) and direct the physiologic processes of the cellular constituents in their area through a process known as nuclear domains. This anatomic arrangement allows segments of the cell to react independently of other portions of the cell. Myonuclei are considered terminally differentiated, with little or no capacity for mitosis and thus for regeneration. Fig. 15-1 Skeletal muscle, isolated intact myofiber. Associated with myofibers are the satellite cells, also known as resting myoblasts (Web Fig. 15-1). These cells are distributed along the length of the myofiber, between the plasma membrane (sarcolemma) and the basal lamina. Satellite cells in skeletal muscle are very different from cells of the same name found within the peripheral nervous system. Muscle satellite cells are fully capable of dividing, fusing, and reforming mature myofibers. Thus, under favorable conditions, muscle cells (myofibers) are able to fully restore themselves after damage. Recent studies have found that pluripotent cells derived from bone marrow can also contribute to skeletal muscle repair, albeit only to a very small degree. Web Fig. 15-1 Skeletal muscle myofibers, transverse section. Each myofiber is surrounded by a basal lamina and outside of this by the endomysium, a thin layer of connective tissue containing capillaries. Myofibers are organized into fascicles surrounded by the perimysium, a slightly more robust layer of connective tissue (Web Fig. 15-2). Entire muscles are encased in the epimysium, a protective fascia that merges with the muscle tendon. This connective tissue framework is not inert, but in fact forms an integral part of the contractile function of muscle by storing and relaying force generated by myofiber contraction. Web Fig. 15-2 Skeletal muscle, transverse section, normal mammalian muscle. Ultrastructural examination reveals that skeletal muscle is a highly and rigidly organized tissue, with what are perhaps the most highly structured cells in the body. Each myofiber is composed of many closely packed myofibrils containing actin and myosin filaments. The striations visible with light microscopy (Fig. 15-2) represent the sarcomeric arrangement of muscle cells, in which actin and myosin filaments attached to transverse Z bands form the framework, and other organelles and intracytoplasmic materials are interspersed within this framework (Fig. 15-3). The endoplasmic reticulum of myofibers is called the sarcoplasmic reticulum and is modified to contain terminal cisternae that sequester the calcium ions necessary to initiate actin and myosin interaction and thus contraction. Sarcolemmal invaginations that traverse the cell, the T (for transverse) tubules, allow rapid dispersion of a sarcolemmal action potential to all portions of the myofiber. The terminal cisternae of two adjacent sarcomeres and the T tubule form what is called the triad (Fig. 15-3, A). Fig. 15-2 Skeletal muscle, longitudinal section, normal mammalian muscle, cytoarchitectural characteristics. Fig. 15-3 Myofiber structure. Neuromuscular junctions can only be visualized using electron microscopy or other specialized procedures (Fig. 15-4). Neuromuscular junctions occur only in specific zones within the muscle, usually forming an irregular circumferential “band” midway between myofiber origin and insertion. Fig. 15-4 Neuromuscular junctions. Mammalian muscles are composed of muscle fibers of different contractile properties. A common classification of these fibers is based on three major physiologic features: (1) rates of contraction (fast or slow), (2) rates of fatigue (fast or slow), and (3) types of metabolism (oxidative, glycolytic, or mixed). These physiologic differences form the basis of histochemical methods that demonstrate fiber types. There are several fiber-type classifications. Classification of fibers into type 1, type 2A, and type 2B (Table 15-1) has proved to have practical application in muscle pathology. It is the classification used in this text. Type 1 fibers are rich in mitochondria, rely heavily on oxidative metabolism, and are slow-contracting and slow-fatiguing. Type 2 fibers have fewer mitochondria and are glycolytic, fast-contracting, and more easily fatigable. In most species, type 2 fibers can be subdivided into type 2A and type 2B. Type 2B fibers are the fast-contracting, fast-fatiguing, glycolytic fibers that depend on glycogen for their energy supply. Type 2A fibers are mixed oxidative-glycolytic and therefore, although fast-contracting, are also slow-fatiguing. Thus type 2A fibers are “intermediate” in the concentration of mitochondria, fat, and glycogen between type 1 and type 2B. TABLE 15-1 Most muscles contain both type 1 and type 2 fibers, and these can be demonstrated by the myosin adenosine triphosphatase (ATPase) reaction (Fig. 15-5, A). Notice that the different fiber types are normally intermingled, forming what is called a mosaic pattern of fiber types. In most mature muscles, the staining pattern of the ATPase reaction reverses when sections are preincubated in an acid rather than an alkaline solution. There are examples of both patterns in the illustrations in this section. Acid preincubation can also be used to distinguish type 2A and type 2B fibers (Fig. 15-5, B). Regenerating fibers, classified as type 2C fibers, stain darkly in both acid and alkaline preparations, which is a distinguishing feature. In most species, oxidative enzyme reactions to demonstrate mitochondria also demonstrate fiber types to some degree (Fig. 15-6, A). Fiber typing can also be done by utilizing immunohistochemical procedures to identify specific myosin isoforms. Fig. 15-5 Muscle fiber typing, myofibrillar adenosine triphosphatase (ATPase) reaction, normal skeletal muscle, transverse section. Fig. 15-6 Mitochondria, NADH reaction (blue stained), skeletal myocytes, normal skeletal muscle, transverse section. The percentage of each fiber type varies from muscle to muscle (Fig. 15-7). Type 1 fibers (slow-contracting, slow-fatiguing, and oxidative) are plentiful in those muscles in which the main function is slow, prolonged activity, such as those that maintain posture. Type 1 predominant postural muscles are most often located deep in the limb. Within the same muscle, the percentage of type 1 fibers often increases in the deeper portions. Muscles that contract quickly and for short periods of time, such as those designed for sprinting, contain more type 2B fibers. Only rarely are muscles composed of only one fiber type (e.g., the ovine vastus intermedius is type 1). Athletic training causes some type 2B fibers to be converted to 2A. There are also variations within breeds and differences in the same muscle in different species. For example, the dog has no type 2B purely glycolytic fibers; all canine fibers have strong oxidative capacity (see Fig. 15-6, B). Fig. 15-7 Schematic diagram of the percentage of type 1 and type 2 myofibers in limb muscles in the dog. The function of skeletal muscle is intimately related to the function of the peripheral nervous system. The physiologic attributes of a muscle fiber—its rate of contraction and type of metabolism (oxidative, anaerobic, or mixed)—are determined not by the muscle cell itself but by the motor neuron responsible for its innervation (Fig. 15-8). This fact is significant in evaluating histologic changes in muscle fibers. It is possible to divide changes in muscle fibers into two major classes: neuropathic and myopathic. Neuropathic changes are those that are determined by the effect or the absence of the nerve supply (e.g., atrophy after denervation). The term myopathy should be reserved for those muscle diseases in which the primary change takes place in the muscle cell, not in the interstitial tissue and not secondary to effects from the nerve supply. The term neuromuscular disease encompasses disorders involving lower motor neurons, peripheral nerves, neuromuscular junctions, and muscles. Fig. 15-8 Schematic diagram of motor units of a muscle. Myofibers require a great deal of energy in the form of adenosine triphosphate (ATP) to generate force and movement. Type 1 oxidative and type 2A oxidative-glycolytic fibers use aerobic metabolism of glucose, stored in the muscle as glycogen, and fat. Type 2B glycolytic fibers rely primarily on anaerobic metabolism of glycogen for energy. Inherent or acquired metabolic defects that reduce skeletal muscle energy production can result in severe muscle dysfunction. A commonly encountered postmortem change, rigor mortis, illustrates the importance of ATP generation within skeletal muscle. The muscle contractile apparatus is still active immediately after death. ATP is necessary for the release of actin from myosin, the interaction that results in the sliding of myofilaments and contraction of muscle. After death, the absence of adequate ATP production causes the muscle fibers to undergo sustained contraction, which is known as rigor mortis. Rigor mortis eventually disappears because of muscle structural breakdown caused by autolysis or putrefaction (bacterial decomposition). The period of time for onset and release of rigor mortis varies, depending on physiologic (glycogen stores at the time of death) and environmental factors such as the environmental temperature (see Chapter 1). Clinical signs of muscular disease are variable (Box 15-1). The most common manifestations are alteration in muscle size, muscle weakness, and abnormal gait. Depending on the nature of the disorder, clinical signs can be localized, multifocal, or generalized. Weakness can be obvious, as in an animal that is unable to rise or prefers to remain recumbent, or can be manifested primarily as exercise intolerance. Special attention should be paid to gait analysis. The gait of an animal with generalized weakness caused by muscle or peripheral nerve dysfunction will have a short stride and often be stiff, and all four legs are often positioned well under the body for support while standing. The abnormal gait of an animal with neuromuscular disease must be distinguished from a similar gait that can occur because of musculoskeletal disease (which is a misnomer, as these disorders affect bone and joint not muscle). Muscle or peripheral nerve dysfunction in the horse, with this species’ unique biomechanics of the pelvic limb, can result in mechanical lameness that can be mistaken for neurologic disease. Odd equine hindlimb gaits designated with such terms as shivers, stringhalt, and fibrotic myopathy are caused by muscle or peripheral nerve disorders. A fibrotic myopathy-like condition also occurs less commonly in the dog and can involve the forelimb. Severe denervating or progressive myopathic conditions that begin in utero or at an early age can cause joint contractures and limb deviation (see Fig. 15-44). Color changes are common. The intensity of the red color of muscle varies, depending on the type of muscle, the age and species of animal, and the extent of blood perfusion. Pale muscle can indicate necrosis (Fig. 15-9, A and B; see Figs. 15-26; 15-34, A; 15-36, A; and 15-40) or denervation (Fig. 15-9, C; see Fig. 15-37) but is also common in young animals and anemic animals. Pale streaking of muscle most often reflects myofiber necrosis and mineralization (see Fig. 15-9, A and B) or infiltration by collagen or fat (see Fig. 15-9, C and D), and is one of the more reliable indicators of gross pathologic changes. Muscle parasites can be grossly visible as discrete, round to oval, pale and slightly firm zones (see Figs. 15-41 and 15-42, A). Dark red mottling of skeletal muscle can indicate congestion, hemorrhage, hemorrhagic necrosis (see Figs. 15-32, A, and 15-38), inflammation, or myoglobin staining after massive muscle damage (see Fig. 15-36, A) or can simply reflect vascular stasis (hypostatic congestion) after death. Hemorrhagic streaks within the diaphragm often accompany death caused by acute exsanguination. A green discoloration can indicate either eosinophilic inflammation (Fig. 15-10) or severe putrefaction. Lipofuscin accumulation in old animals, especially cattle, can cause a tan-brown discoloration of muscle. Black discoloration of the fascia occurs in calves with melanosis as an incidental finding and in older gray horses with metastasis of dermal melanoma to muscle fascia. Fig. 15-9 Pathologic changes resulting in pale skeletal muscle. Fig. 15-10 Bovine eosinophilic myositis, gluteal muscles, cow. Contraction of muscle after contact with fixative is the most common cause of an artefact called contraction band artefact. Contraction can be prevented or at least minimized by use of a specially designed muscle clamp (Web Fig. 15-3, A) or by placing the sample on a rigid surface, such as a portion of a tongue depressor, and fixing the ends with sutures, staples, or clamps before submersion in the fixative (Web Fig. 15-3, B). Web Fig. 15-3 Techniques for collection of muscle samples for histologic examination. Frequently, lesions in muscles can be detected and evaluated only by microscopic examination. Proper microscopic examination requires evaluation of both transverse and longitudinal sections. Myofiber diameters, cytoarchitectural changes, and the percentage of abnormal myofibers are most reliably evaluated in transverse sections. Longitudinal sections reveal the length of changes such as segmental necrosis or regeneration or deposition of storage material. Improperly oriented samples, which result in sections that have obliquely oriented myofibers and thus neither longitudinal nor transverse myofibers, are difficult to evaluate. Use of a magnifying glass or dissecting microscope can aid in determining the orientation of myofibers during trimming of muscle before sectioning. Routine stains, such as hematoxylin and eosin (H&E), run the risk of offering the pathologist a “vast pink wasteland” for evaluation (Fig. 15-11, A) and are often inadequate for detecting subtle myopathic changes, lesions within intramuscular nerves, or presence of abnormal stored material. Various special stains, including reticulin, Masson trichrome, von Kossa, lipid (performed on frozen sections of fixed samples), and periodic acid–Schiff (PAS) for glycogen, are often invaluable in evaluation of routinely processed skeletal muscle (Web Table 15-1). Examples of many of these disorders can be found here in this chapter. Other valuable stains and reactions can only be performed on frozen sections of unfixed muscle samples (see Web Table 15-1). Fig. 15-11 Visibility of mitochondria, formalin-fixed vs. frozen sections, transverse sections of skeletal muscle. Enzyme Histochemistry and Immunohistochemistry: There is no question that frozen section histochemistry of unfixed muscle samples is the “gold standard” of muscle pathology. Skeletal muscle may be the one tissue in which the morphology of cells and cellular components is best appreciated in frozen sections (Fig. 15-11, B). Routine frozen section histochemistry on muscle includes a battery of stains applied to serial sections. Examples of many of these stains are illustrated in this chapter. Stains used include H&E, modified Gomori’s trichrome, ATPase for fiber typing, nicotinamide adenine dinucleotide dehydrogenase (NADH), succinate dehydrogenase (SDH), cytochrome oxidase, and other mitochondrial enzyme stains, PAS for glycogen, alizarin red S for calcium, alkaline phosphatase and nonspecific esterase for macrophages and denervated fibers, and lipid stains. When indicated, frozen sections also allow for immunostaining for cytoskeletal proteins, such as dystrophin (see Fig. 15-46) and the dystrophin-associated proteins. Certain abnormal structures, such as nemaline rods formed by expansion of Z bands, as seen in nemaline rod myopathy, are not visible in routine sections but are readily identified in frozen sections stained with modified Gomori’s trichrome. Electron Microscopy: Although much of what used to be determined by electron microscopy has been supplanted by newer immunohistochemical procedures, electron microscopic evaluation of muscle is still important. Various structural alterations, such as abnormalities of neuromuscular junctions, mitochondria, sarcomeric disarray, sarcotubular dilation, Z-line streaming, and cytoplasmic inclusions, may be best visualized, and in some cases only visualized, by this method. Sampling and handling methods to minimize contraction and other artefacts and to allow for precise transverse and longitudinal sections are imperative. Portals of entry are summarized in Box 15-2. Injury to muscle can occur secondary to trauma or infection. Muscle lying superficially can be damaged by penetrating wounds, including those created by intramuscular injections (Fig. 15-12; see also Fig. 15-9, B), which can also allow entry of infectious agents. Muscles located deeply are often injured after bone fracture. Crush injuries from external forces cause extensive muscle damage, and excessive tension can cause muscle tearing. Muscles are endowed with an extensive vascular network that can allow entry of blood-borne pathogens, immune complexes, antibodies and toxins, and inflammatory cells. Fig. 15-12 Inflammation and myofiber necrosis, injection site, muscles, lateral thigh, cow.
Skeletal Muscle
Structure
Structure of Myofibers
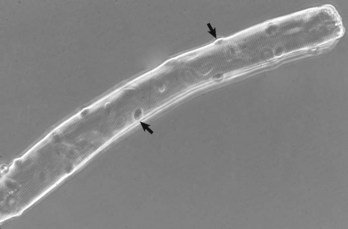
Note the multiple peripherally located nuclei (arrows). Phase contrast microscopy. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
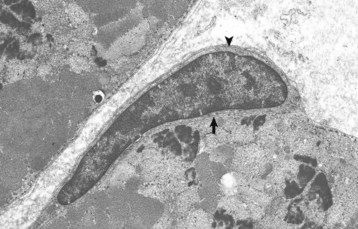
Note the satellite cell (resting myoblast) located between the sarcolemma (arrow) and the basal lamina (arrowhead). TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
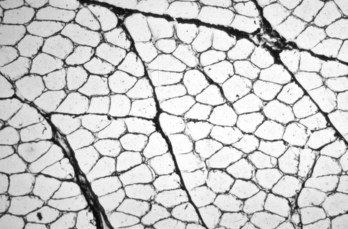
Each myofiber is surrounded by an endomysium of fine collagenous connective tissue. Myofibers are organized into fascicles, which are surrounded by a slightly thicker perimysium. Frozen section, reticulin stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
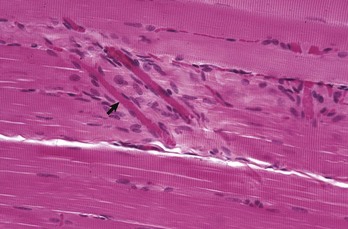
Note the peripherally located myofiber nuclei and cross striations on the muscle fibers. The cross striations correspond to the A bands (dark lines) and I bands (light lines) in the transmission electron micrograph of Fig. 15-3, B. Myofibers are surrounded by an extensive capillary network (arrow). Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
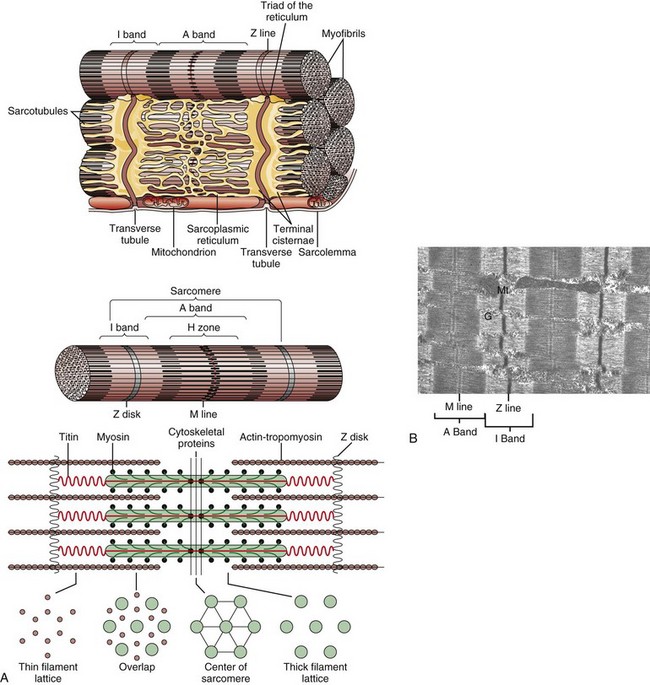
A, Schematic representation of myofiber orientation, secondary organelles, and ultrastructural arrangement of cytoskeletal proteins within sarcomeres. B, Skeletal muscle, longitudinal section, normal mammalian skeletal muscle. Sarcomeres are defined by Z lines, A bands composed of thick myosin filaments, and I bands composed of thin actin filaments. Dense M lines with adjacent clear H zones occur in the center of the A band. Mitochondria (Mt) and glycogen (G) are interspersed between the myofibrils. TEM. uranyl acetate and lead citrate stain. (A from Copstead-Kirkhorn LE, Banasik JL: Pathophysiology: biological and behavioral perspective, ed 3, St Louis, 2005, Saunders. B courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
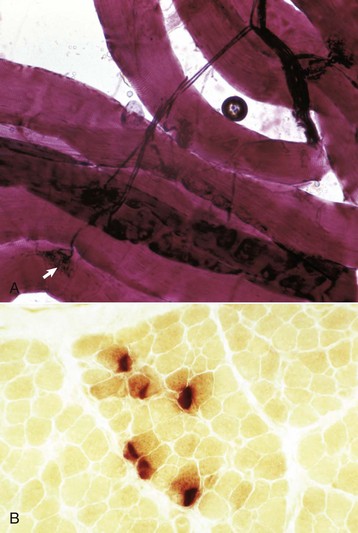
A, An intramuscular nerve (top right) has given off axons, which terminate on a myofiber at a neuromuscular junction (arrow). Teased preparation, silver impregnation method. B, Neuromuscular junctions, transverse section through the center region of normal mammalian muscle. The neuromuscular junctions (red-brown stain) form a cluster. Nonspecific esterase stain, frozen section. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Types of Myofibers
Fiber Type
Physiologic Characteristics
Morphologic Characteristics
1
Slow twitch, oxidative, fatigue resistant, “red muscle,” aerobic
High mitochondrial content, high fat content, low glycogen content
2A
Fast twitch, oxidative and glycolytic, fatigue resistant
Intermediate mitochondria, fat, and glycogen content
2B
Fast twitch, fatigue sensitive, glycolytic, “white muscle,” anaerobic
Low mitochondrial and fat content, high glycogen content
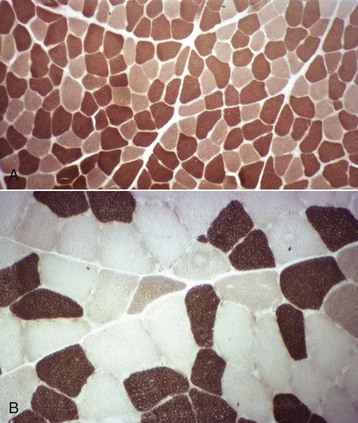
A, Dog. Type 1 (light) and type 2 (dark) fibers are arranged in a mosaic pattern. Frozen section, ATPase pH 10.0. B, Horse. Acid preincubation allows differentiation of three fiber types, type 1 (dark), type 2A (light), and type 2B (intermediate = gray). Frozen section, ATPase 4.35. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
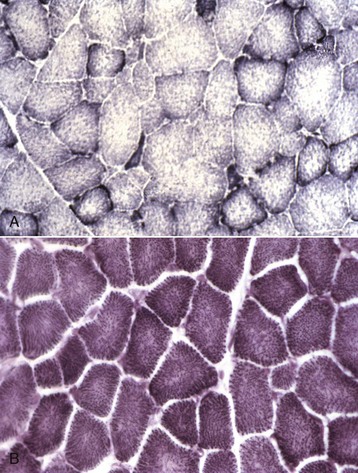
A, Horse. Type 1 fibers contain the most mitochondria, type 2B the least, and mitochondrial content of type 2A fibers is intermediate between type 1 and type 2B. Frozen section, NADH reaction. B, Dog. All fiber types have a similar mitochondrial content, therefore this reaction cannot be used to identify different types of myofibers in canine muscle. Frozen section, NADH reaction. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
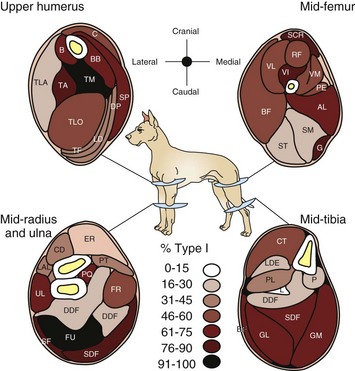
There is a wide variation from muscle to muscle. Deeply located muscles have the most type 1 myofibers, indicative of their function in maintaining posture. (Redrawn from Armstrong RB, Sauber CW, Seeherman HJ, Taylor CR: Am J Anat 163:87-98, 1987.)
Function
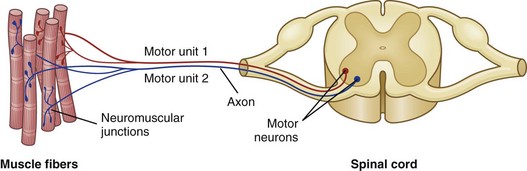
Each motor unit consists of a motor neuron within the central nervous system and all the myofibers (muscle cells) supplied by the neuron and its axon branches. (From Huether SE, McCance KL: Understanding pathophysiology, ed 4, St Louis, 2008, Mosby.)
Metabolism and Ionic Homeostasis
Examination of Muscle: Clinical, Gross, and Microscopic
Clinical Findings
Methods of Gross and Microscopic Examination of Muscle
Gross Pathology and Muscle Sampling
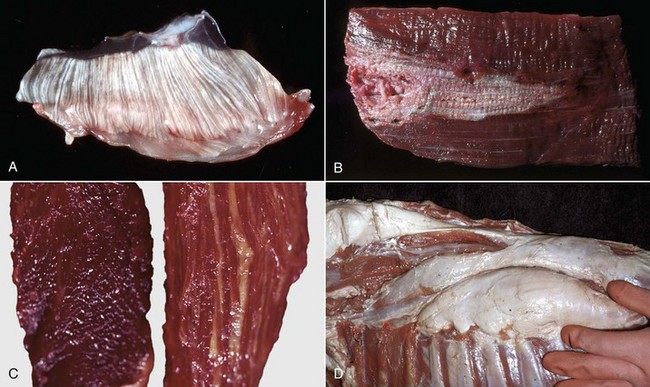
A, Pale streaks, necrosis and mineralization, degenerative myopathy, canine X-linked muscular dystrophy, diaphragm (left side), dog. B, Localized pallor, necrosis, injection site of an irritant substance, semitendinosus muscle, cow. The irritant was injected just under the perimysium and caused necrosis and disruption of the myofibers. Some irritant seeped down between the fascicles to cause necrosis, but the fascicles of myofibers are still in place. C, Overall pale muscle with pale streaks from collagen and fat infiltration, denervation atrophy, equine motor neuron disease, horse. Equine motor neuron disease muscle (right) compared with normal muscle (left). D, Enlargement and pallor, steatosis, longissimus muscles, neonatal calf. The majority of the muscles have been replaced by fat. (A courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University. For histopathologic findings, see Fig. 15-45. B and D courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. For histopathologic findings, see Fig. 15-25. C courtesy Dr. A. de Lahunta, College of Veterinary Medicine, Cornell University. For histopathologic findings, see Figs. 15-19 and 15-37.)
Green discoloration of the muscle is due to inflammation that has abundant eosinophils. The inflammation is attributed to degenerating Sarcocystis spp. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. For histopathologic findings, see Fig. 15-39.)
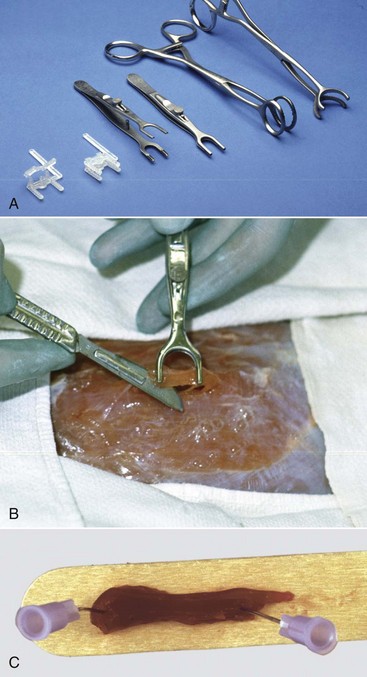
Clamps are used to prevent contraction of a fresh muscle specimen when it is immersed in 10% neutral-buffered formalin or EM fixative. A, Types of muscle clamps (from left to right): disposable plastic clamps (open and closed), stainless steel clamps (open and closed), a gallbladder clamp (unmodified), and a modified gallbladder clamp. The stainless steel clamps are autoclavable, the best but expensive. A suitable and economical clamp (not shown), can be made by welding a bar approximately 1 cm long, 3 to 5 mm wide, and 3 mm thick between the lower jaws of two small hemostats. B, Final excision stage. Initially two longitudinal incisions, approximately 5 mm apart and 15 mm long are made into the muscle in the direction of the myofibers. A horizontal cut is made 3 to 4 mm below the surface to undermine a piece of muscle. One jaw of the clamp is inserted under the muscle until its tip just exits on the other side. The clamp is lifted several mm above the surface of the muscle to ensure that the muscle fibers are tense and then the jaws are clamped. The clamped piece of muscle is excised by cutting at each end adjacent to the clamp as shown above. The muscle sample, still in the clamps, is placed in the fixative, usually 10% BNF for histopathologic examination and fixed overnight. For fixation for electron microscopic examination, the muscle in the clamp is placed into EM fixative for 1 to 2 hours. For histopathologic examination the muscle is trimmed by freeing the strip of muscle between the clamps by cutting immediately adjacent to the clamp jaws. Then a transverse section is cut from one end of this sample, avoiding any crushed area, and the remainder of the sample is cut longitudinally in the direction of the myofibers. Both samples are desirable for histopathologic examination. For electron microscopy, after fixation for 1 to 2 hours, slivers 0.5 to 1 mm thick are shaved from the outside of the sample. These are cut into pieces 0.2 mm in diameter and 0.5 mm long, with the longer dimension being in the direction of the myofibers. This long sample facilitates embedment so that the fibers are oriented either in cross section or longitudinally. Both sections are required for electron microscopy. C, Pinning strips of muscle onto a rigid surface, such as a piece of tongue depressor before immersion in 10% neutral-buffered formalin, will also minimize fixation artifacts but is not as effective as the clamps shown above. (A and B courtesy Dr M.D. McGavin, College of Veterinary Medicine, University of Tennessee. C courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Microscopic Examination
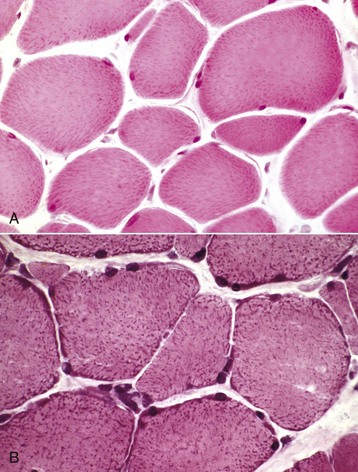
A, Formalin-fixed, paraffin-embedded sample. Mitochondria stain faintly, and the myofibers lack detail when compared with B. H&E stain. B, Frozen section. Note the increased detail visible. Blue-stained mitochondria are seen throughout the cytoplasm and concentrated at the periphery of the myofibers. H&E stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Portals of Entry
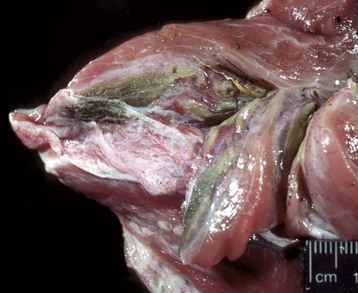
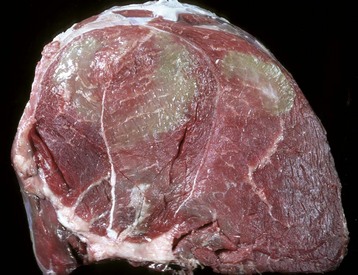
Necrotic muscle has been stained green by the injected material, which has spread distally down the fascial plane between the two muscles from the original injection site (top right). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
