Michael T. Walsh, Martine de Wit
Sirenia
The Florida manatee (Trichechus manatus latirostris), a member of the Trichechidae family of the order Sirenia, will be used to illustrate topics in Sirenia medicine and surgery for this chapter. When possible, the Antillean manatee (Trichechus manatus manatus) and the Amazonian manatee (Trichechus inunguis) will be included, although material on these and the West African manatee (Trichechus senegalensis) is sparse. Data will be used from the only surviving member of the family Dugongidae, the Dugong (Dugong dugon), with the help of colleagues in Australia.
Sirenia are found in over 80 countries and territories throughout the tropical and subtropical latitudes.29 These marine mammal herbivores inhabit coastal waters and river systems. Their range is generally restricted by water temperatures, and both dugongs and Florida manatees, which are at their outer range for comfort, may migrate to warmer water during their respective winter periods.
There has been a great increase in effort in all Sirenia species to collect ecologic and health information as habitats are placed under continuing pressure from anthropogenic and natural factors. The natural history, ecology, and genetic background of these species is covered in a number of publications, and the interested reader is referred to these for additional information.2,9,29,35,36,45 A valuable source of information on Sirenia is available online in an extensive bibliography compiled by Daryl P. Domning, Bibliography and Index of the Sirenia and Desmostylia (http://www.sirenian.org/biblio/).14 Additional material (and useful videos) on Sirenia health will be available to the reader in the future at http://aquaticanimalhealth.org/multimedia.html.
Clinically Important Anatomy
Anatomy investigations may be traced back to the 1870s.33 Sirenians are fusiform in shape with very sensitive facial hairs and prehensile capability to acquire vegetation for ingestion. The eyes are generally small, and the external auditory meatus is about 1 millimeter (mm) in diameter along the side of the skull. The variation in the angle of the rostral portion of the skulls between manatees and dugongs is based on their feeding habits. The dugong has prominent incisor teeth (tusks) present in both genders. Sirenia pectoral flippers are flattened laterally and medially, have three to four nails, and may assist in maneuvering, pulling along the bottom during feeding, and tactile contact between animals, including manipulation of females by males whose flippers are longer and rougher medially to hold on during breeding activity. The nipples are placed in the axillary region, as in elephants, and testes are internal. The tail or paddle is round in manatees, and the dugong tail is shaped like that of a dolphin, with symmetrical fluke blades extending laterally from the vertebral attachment. Each lung consists of an elongated single lobe placed in its own pleural cavity created by a midline connection of the hemi-diaphragms to the vertebral bodies. The cranial 70% of each manatee lung attaches medially at the vertebrae. The heart is cranioventral to the pleural cavities and separated from the abdominal cavity by a transverse septum. The liver and intestinal tract lie ventral to the hemi-diaphragms. Manatees are hindgut fermenters, and the gastrointestinal (GI) tract may make up to 23% of an animal’s body weight,37 exhibiting a number of unique features. The stomach is a single C-shaped sac and has a digestive cardiac gland. The duodenum has three portions, including the enlarged duodenal ampulla, the two duodenal diverticula, and the post-ampulla duodenum. The cecum is rounded with two accessory sacs, giving it a rabbit-ear appearance. The kidneys are multilobulated, retroperitoneal, and caudal to the lungs. The vulva is located just anterior to the anus with a circular rim of tissue. The distal portion of the vagina is vertical, with the urethral opening located deep and inaccessible for catheterization unless the animal is heavily sedated. The penile opening is located on the ventral midline and is anterior toward the umbilicus. Vascular access for catheterization is hampered by the presence of vessel bundles in the accessible areas of the flipper and the caudal intervertebral area.
Veterinary Interaction with Sirenia
A number of book chapters and articles cover a range of Sirenia health issues, treatments, and pathologic findings7,34,42,45 Veterinary involvement with manatees occurs in rescues, research-based health assessments, health assessments of animals being monitored after release, rehabilitation, long-term management in aquaria and zoologic institutions, and necropsies. The chapter will focus on these areas, with references provided where the authors do not have first-hand knowledge of the activity described.
Rescue, Transport, and Rehabilitation
Manatees and dugongs may require intervention for a number of diseases and anthropogenic challenges.24 First response is usually performed by state or facility personnel with experience in manatee capture and transport. Facility veterinarians may be present for the operation or are in contact with rescuers by phone. The most common reasons for rescue and rehabilitation include cold stress, orphaned calves, other natural disease (reproductive complications, biotoxins, infectious disease, noninfectious disease), watercraft injuries, entrapment, and other human-related etiologies such as entanglement or ingestion of fishing gear. Observation of the ill or injured manatee’s behavior prior to capture may be very helpful in understanding the cause of the problem and preparing for transport and therapy. New availability of above-water and underwater cameras placed at winter congregation sites may improve access for visual evaluation of ill and injured animals at specific sites. Cardiopulmonary compromise from pneumonia, fractures, pneumothorax, or pyothorax may require special handling to decrease further damage. If pneumothorax and broken ribs are suspected, attempts should be made to reduce further damage to the lung and the surrounding tissue from manipulation, loading, and transport.
Critical Care Facility Medicine and Management
At the critical care facility, manatees undergo a visual inspection of body condition, eyes, oral cavity, wounds or lesions, and respiratory rate and quality, heart rate, and oral temperature are recorded. Thin or emaciated animals should have a blood sample taken early in the evaluation to include complete blood cell count (CBC), chemistries, spun packed cell volume (PCV), total protein levels, and glucometer glucose levels before placing the animal in water. CBC count and chemistry values are listed in Table 45-1 and 45-2.22,23 Standard blood sampling for manatees is from the interosseous space between the radius and the ulna, which may be approached medially or laterally. The site is visually defined by flexing the carpal joint and noting the elbow joint and then palpating the caudal edge of the radius between the joints. The medial insertion site may strike the median nerve and create discomfort in and resistance from the animal after multiple blood samples. The lateral approach may result in less discomfort but the vessel location is (goes) deeper, and redirection of the needle or obtaining large volumes is more difficult. Small calves may be bled with 25- to 23-gauge needles with small extension sets; however, rapid clotting may occur, so having (using) preheparinized syringes may help avoid sample loss. One-inch (25-mm), 20-gauge needles with extension sets are used in juveniles. Blood samples may be obtained from adults with 1.5-inch (38-mm) 20- to 18-gauge needles attached to extension sets, which helps avoid needle displacement when the animal moves. Cytology and cultures of lesions may help guide therapy. Absence or presence of a scant amount of dry, hard feces may suggest dehydration and constipation. Hypoglycemic animals should receive glucose supplementation and oral fluids, and a constipated animal’s oral fluid should be lubricated with mineral oil prior to release of the animal into a pool. Gavage hydration or feeding is always done last to avoid possible complications related to handling and regurgitation. Additional diagnostic techniques may be delayed, and the priority should be to place the animal back into water after an extended transport.
TABLE 45-1
Hematologic Results for Free-Ranging and Captive Manatees (Trichechus manatus)*
| Analyte | Free-Ranging (n = 52) | Captive (n = 62) | P Value |
| Hematocrit, liter per liter (L/L) | 0.351 (0.289–0.435) | 0.350 (0.277–0.461) | 0.731 |
| Hemoglobin, gram per liter (g/L) | 112 (94–135) | 112 (86–149) | 0.784 |
| Red blood cell count (×1012/L) | 2.76 (2.17–3.39) | 2.81 (2.30–3.51) | 0.366 |
| Mean corpuscular volume, (fL) | 128 (114–140) | 124 (105–140) | 0.003 |
| Mean corpuscular hemoglobin, picogram (pg) | 40.8 (36.6–44.9) | 40.0 (32.9–44.3) | 0.015 |
| Mean corpuscular hemoglobin concentration (g/L) | 320 (280–354) | 320 (294–336) | 0.973 |
| Red cell distribution width (%) | 16.2 (13.9–22.8) | 16.6 (13.5–21.4) | 0.139 |
| Platelets (×109/L) | 242 (111–424) | 274 (137–507) | 0.051 |
| Mean platelet volume (fL) | 6.44 (5.02–8.49) | 6.40 (5.19–8.80) | 0.625 |
| White blood cell count (×109/L) | 5.98 (2.77–13.50) | 6.64 (2.85–14.2) | 0.070 |
| Bands (×109/L) | 0.02 (0–0.22) | 0.02 (0–0.16) | 0.912 |
| Heterophils (×109/L) | 2.33 (0.77–6.53) | 3.25 (1.40–8.50) | <0.001 |
| Lymphocytes (×109/L) | 2.90 (1.01–7.20) | 2.71 (0.83–8.50) | 0.811 |
| Monocytes (×109/L) | 0.51 (0.08–1.70) | 0.56 (0.07–2.80) | .537 |
| Eosinophils (×109/L) | 0.20 (0–1.23) | 0.09 (0–0.41) | <0.001 |
| Basophils (×109/L) | 0.04 (0–0.27) | 0.03 (0–0.14) | 0.138 |
| Nucleated red blood cell (×109/L) | 0.02 (0–0.21) | 0.04 (0–0.25) | 0.028 |
| Reticulocytes, manual (×109/L)† | 51 (16–96) | 74 (0–127) | 0.076 |
| Reticulocytes, automated (×109/L)‡ | 17 (7–45) | 21 (3–39) | 0.424 |
| Heinz bodies (%)§ | 8.2 (0.6–22.3) | 7.0 (0–15) | 0.181 |
| Plasma proteins (g/L) | 80 (69–93) | 81 (70–92) | 0.641 |
| Fibrinogen (g/L) | 2.24 (1.00–5.00) | 3.23 (1.00–6.00) | 0.002 |
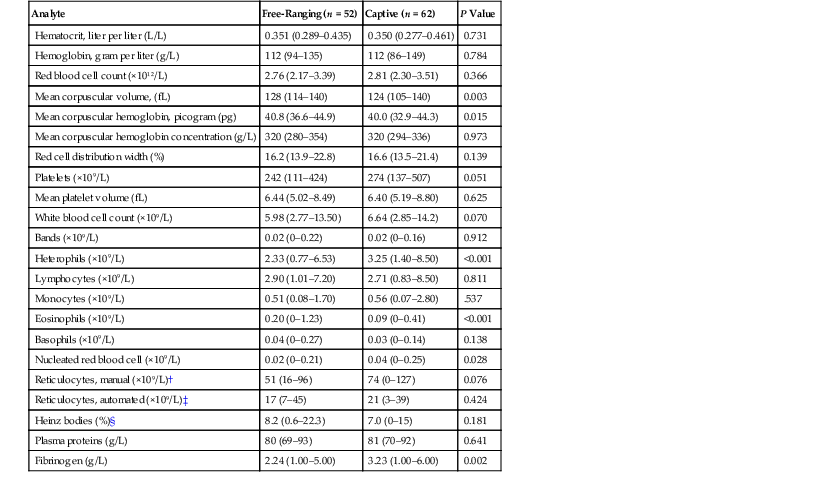
* Data are mean (minimum–maximum). Data for small calves were not included. Except for reticulocytes, P values were determined by comparing location (free-ranging versus captive) and age of blood sample (fresh versus day old) using a two-way ANOVA (analysis of variance) with the Tukey test for group comparisons. Only fresh blood samples were analyzed for reticulocyte counts, so P values were determined for free-ranging versus captive animals using a t-test.
† n = 17 free-ranging, 15 captive
‡ n = 10 free-ranging, 9 captive
§ n = 28 free-ranging, 40 captive
Table reproduced with permission from Harvey JW, Harr KE, Murphy D, et al: Hematology of healthy Florida manatees (Trichechus manatus). Vet Clin Pathol 38(2):183–193, 2009.
TABLE 45-2
Plasma Clinical Biochemistry Analytes in Free-Ranging and Captive Trichechus Manatus*
| Analyte | Free-Ranging | Captive | P Value |
| Sodium, millimole per liter (mmol/L) | 150 (143–158) | 150 (142–161) | P = 0.30 |
| Potassium (mmol/L) | 4.9 (3.8–6.3) | 4.8 (3.5–6.7) | P = 0.23 |
| Chloride (mmol/L) | 93 (78–106) | 86 (71–98) | P <0.001 |
| Total carbon dioxide (mmol/L) | 27 (4–41) | 40 (14–54) | P <0.001 |
| Anion gap (mmol/L) | 39 (15–59) | 28 (5–46) | P <0.001 |
| Lactate (mmol/L) | 13 (1–30) | 4 (1–24) | P = 0.002 |
| Calcium (mmol/L) | 2.6 (2.0–3.7) | 2.8 (2.2–3.4) | P = 0.03 |
| Phosphate (mmol/L) | 1.9 (1.1–2.7) | 1.6 (1.1–2.9) | P = 0.03 |
| Magnesium (mmol/L) | 1.9 (1.2–3.1) | 1.4 (0.7–1.9) | P <0.001 |
| Urea (mmol/L) | 2.1 (0.4–4.3) | 4.6 (1.8–8.9) | P <0.001 |
| Creatinine, micromole per liter (µmol/L) | 128 (53–336) | 194 (115–345) | P <0.001 |
| Bilirubin (µmol/L) | 1.7 (0–5.1) | 1.7 (0–8.6) | P = 0.88 |
| Glucose (mmol/L) | 4.6 (2.3–9.9) | 4.4 (1.9–8.6) | P = 0.82 |
| Cholesterol (mmol/L) | 3.0 (2.1–7.3) | 3.5 (1.9–7.6) | P = 0.91 |
| Triglycerides (mmol/L) | 0.9 (0.3–2.2) | 0.8 (0.3–1.6) | P = 0.04 |
| ALP, unit per liter (Unit/L) | 86 (39–192) | 122 (51–242) | P <0.001 |
| GGT (Unit /L) | 31 (9–47) | 46 (11–99) | P <0.001 |
| ALT (Unit /L) | 18 (5–48) | 17 (1–94) | P = 0.52 |
| AST (Unit /L) | 9 (4–26) | 9 (3–37) | P = 0.60 |
| CK (Unit /L) | 190 (51–2966) | 261 (64–3348) | P = 0.64 |
| Total protein, gram per liter (g/L) | 76 (64–90) | 80 (71–93) | P <0.001 |
| Albumin (g/L) | 34 (25–46) | 39 (33–47) | P <0.001 |
| Total globulins (g/dL) | 41 (33–54) | 43 (32–58) | P = 0.96 |
| A/G ratio | 0.86 (0.61–1.31) | 0.89 (0.58–1.33) | P = 0.006 |
| Haptoglobin (g/L) | 1.5 (0.3–2.5) | 0.9 (0.2–2.1) | P = 0.04 |
| SAA, milligram per liter (mg/L) | 11 (<10–50) | 13 (<10–49) | P = 0.91 |
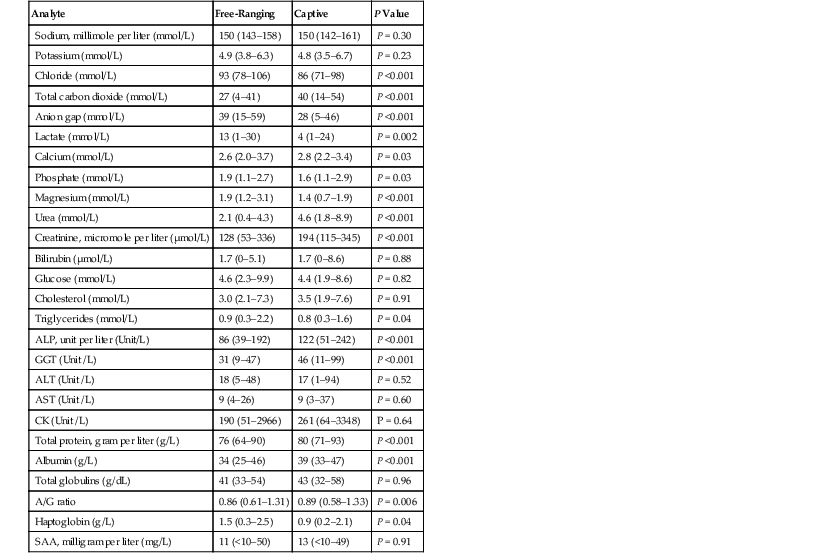
* Values are medians with minimum and maximum values in parentheses. The total number of manatees analyzed for a majority of analytes was 112, but lesser numbers were analyzed for total carbon dioxide and anion gap (98), cholesterol, albumin, globulin, and A/G ratio (86), ALT, AST, and haptoglobin (72), and lactate (61). Albumin and total globulins were determined using plasma protein electrophoresis. Data for small calves were not included. P values were determined by comparing location and age classes using a two-way ANOVA (analysis of variance) with the Tukey test used for comparison of free-ranging and captive animals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


