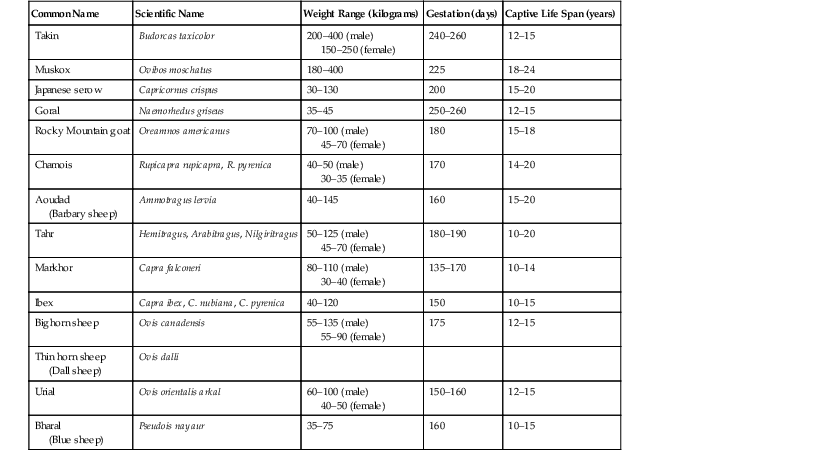
Martha A. Weber Wild sheep and goats belong in the family Bovidae and are grouped in the subfamily Caprinae. The three tribes within the Caprinae are (1) Ovibini (muskox and takin), (2) Rupricaprini (goral, serow, and chamois), and (3) Caprini (sheep, goat, and tahr). Eighty-five species and subspecies are recognized by the International Union for the Conservation of Nature (IUCN); 63% of these are categorized as Near Threatened, Vulnerable, Endangered, or Critically Endangered. Overhunting, habitat loss, and disease transfer from domestic sheep and goats are responsible for many population declines. Caprinae species are distributed across the northern hemisphere, often in inhospitable environments such as deserts, tundra, mountains, or forests. Generally, both sexes have horns; depending on the species, these may be short and sharp or large and ornate. Marked sexual dimorphism exists in the size and shape of the horns in animals in the tribe Caprini; this is less distinct in the other tribes. Table 64-1 lists characteristics for selected species. Global climate change may hasten the population decline or extinction of some species. Cold-adapted animals may experience increased stress in the face of local temperature changes. Movement of animals out of their traditional ranges, exposure to novel pathogens as species commingle, and changes in parasite and vector dispersal could all play a role in the development of new epizootic diseases in populations. Most Caprinae are agile climbers and jumpers. Enclosures and holding areas need adequate moats, fencing, or both to prevent escape. Climbing structures promote natural behaviors and hoof wear in montane species, but it must be ensured that they do not provide a point from which an animal can leap out of the enclosure. Species adapted to northern climates should have access to shade structures and cooling sprays when housed in warm and humid environments. Caprinae are ruminants and, in the wild, may be grazers, browsers, or generalist herbivores. Diets in captivity generally consist of concentrate pellets and grass or alfalfa hay. Salt blocks may be provided. Some species may be sensitive to high levels of dietary copper, so trace mineral blocks should not be used at all or used with caution. Food items should be presented in multiple locations around an enclosure to keep dominant animals from preventing others access to food. Nontoxic browse items may be added to the diet as they are available. Free-ranging animals may be manually captured with drive nets or box traps. When herding animals, it is important to be aware of hazards such as rock faces, precipices, or water. Prolonged running may induce hyperthermia and capture myopathy. Acepromazine, azaperone, xylazine, and haloperidol have been used, with variable results, to provide sedation for wild chamoises and ibexes that were restrained for several hours after capture.4,20,22 Some smaller species in captivity (e.g., urial, goral) may be hand captured and restrained for short procedures (hoof trim, venipuncture, tuberculin testing). Handlers need to take into consideration that the animals may jump, bank off walls, and inflict injury with their horns. The horns of juvenile animals should not be handled for restraint, as the bony core may not be fused to the skull and thus may break. Blindfolding restrained animals may help keep them calm during the procedure. Respiratory rate and body temperature should be monitored during restraint. General anesthesia may be used for large species, invasive procedures, or procedures of longer duration. Commonly used chemical restraint agents in Caprinae include opioids, dissociative agents, and α2-agonists.5 Opioids used include the ultrapotent narcotic agents carfentanil, thiafentanil, and etorphine. These agents are highly regulated in some countries and do pose a risk to personnel who may come into contact with the substances. The use of butorphanol, a partial opiate agonist–antagonist, in combination with α2-agonists, has been reported to result in excellent anesthesia in takins, with a significant decrease in rumen reflux.23 Reversal of opiate anesthesia is generally achieved using naltrexone administered intramuscularly (IM) or intravenously (IV). α2-agonists, including xylazine, medetomidine, and detomidine, have been used in Caprinae. Medetomidine and detomidine bind more specifically to the α2-receptors than xylazine, resulting in greater potency. Atipamezole is recommended for reversal, although yohimbine or tolazoline may also be used to reverse the effects of xylazine. The dissociative anesthetic agents ketamine and tiletamine–zolazepam are also commonly used in Caprinae. Good quality anesthesia is usually obtained with these agents, in combination with α2-agonists or opiates. Ketamine and tiletamine are not reversible, which may be a disadvantage when immobilizing free-ranging specimens. Supplemental ketamine may be given intravenously, either as boluses or a constant rate infusion, to enhance or prolong anesthesia. Induction doses are generally administered intramuscularly by hand syringe, pole syringe, or projectile dart. If an animal is restrained manually or in a chute intravenous injection may be an option. Intubation is recommended for all animals that are fully anesthetized to prevent aspiration of refluxed rumen contents. The use of stylets greatly facilitates intubation in species with narrow muzzles. Isoflurane works well to maintain anesthesia for procedures of long duration. The published literature should be reviewed to identify current dosage recommendations for anesthetic agents and species-specific factors. Physical examination is performed as in other ruminant species. Body condition should be assessed regularly. Blood is generally collected from the jugular vein. Hematologic and biochemical parameters for selected species are listed in Tables 64-2 and 64-3. Hematocrit values from automated cell counters are often very low in takins and a spun packed cell volume (PCV) should be measured as well. TABLE 64-2 Reference Ranges for Hematologic Parameters of Selected Caprinae Species
Sheep, Goats, and Goat-Like Animals
Biology
Special Housing Requirements
Feeding
Restraint and Handling
Chemical Restraint and Anesthesia
Diagnostics
Parameter
Takin (Budorcas taxicolor)
Goral (Naemorhedus griseus)
Urial (Ovis aries arkal)
Domestic Goat (Capra hircus)
Erythrocytes ( ×106/ml)
8.0–14.0
7.8–13.5
10.7–14.5
9.3–14.7
Packed cell volume (%)
25–35
27–45
39–50
26–39
Hemoglobin (g/dl)
9.1–12.3
9.4–15.8
12.3– 5.5
9.2–13.3
Leukocytes (per ml)
4900–12,000
2400–6,300
3500–8000
4500–12,300
Neutrophils (per ml)
3200–8500
1100–3,200
1000–4700
2400–8800
Lymphocytes (per ml)
1500–2500
750–2,500
1000–4000
2100–7300
Monocytes (per ml)
100–300
0–300
0–300
150–400
Eosinophils (per ml)
0–100
0–100
0–200
0–400
Basophils (per ml)
0–50
0–50
0–50
0–200
Platelets (x 103/ml)
250–700
250–900
300–750
200–700
Fibrinogen (mg/dl)
100–500
100–500
200–500
250–500 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sheep, Goats, and Goat-Like Animals
Chapter 64