A. Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following:
1. Eating, in a discrete period of time (e.g., within any 2-h period), an amount of food that is definitely larger than most people would eat during a similar period of time and under similar circumstances.
2. A sense of lack of control over eating during the episode (e.g., a feeling that one cannot stop eating or control what or how much one is eating).
B. Recurrent inappropriate compensatory behavior in order to prevent weight gain, such as self-induced vomiting; misuse of laxatives, diuretics, enemas, or other medications; fasting; or excessive exercise.
C. The binge eating and inappropriate compensatory behaviors both occur, on average, at least twice a week for 3 months.
D. Self-evaluation is unduly influenced by body shape and weight.
E. The disturbance does not occur exclusively during episodes of Anorexia Nervosa.
Purging Type: during the current episode of Bulimia Nervosa, the person has regularly engaged in self-induced vomiting or the misuse of laxatives, diuretics, or enemas.
Nonpurging Type: during the current episode of Bulimia Nervosa, the person has used other inappropriate compensatory behaviors, such as fasting or excessive exercise, but has not regularly engaged in self-induced vomiting or the misuse of laxatives, diuretics, or enemas.
Additional diagnostic features of BN are excessive concern about food, body weight, and body shape. Patients are typically normal weight: emaciation takes diagnostic priority and thus an underweight individual exhibiting binge eating and purging would be considered to have anorexia nervosa (AN). Historically, only a small proportion of patients with BN have been found to be overweight (e.g., 4.2% in one 1990 study (8)), though in a recent study, the prevalence of overweight people in a community-residing population with BN was 64% (9). Thus, the percentage of individuals with BN who are overweight or obese is not well established.
Risk factors linked to the development of BN include a history of parental obesity or of childhood obesity, early parental criticism about eating and body weight (10, 11), dieting, and a history of AN (12). Initiating factors for the disorder may include any of a number of circumstances that precipitate dieting behavior or dietary restriction. Dieting usually precedes binge eating, though a significant subgroup reports binge eating prior to the onset of dieting (13). As with other eating disorders, factors that maintain the behavioral cycle of BN may be distinct from factors that initiate BN (14).
Factors likely to initiate a binge meal and thus perpetuate the behavioral cycle of BN include cognitive, affective, and learned factors such as binge-eating episodes preceded by negative mood states. This suggests that some binge episodes are attempts to relieve such aversive states. Learned factors probably play a prominent role in binge eating. For example, there is behavioral (15) and self-reported (16) evidence for heightened responsiveness to binge food stimuli, “cue reactivity,” in this population. Dietary restriction in between binge-eating episodes is common, and resultant hunger may serve to “fuel” subsequent overeating (17, 18). A typical pattern of eating may feature skipping breakfast, consuming a minimal lunch, and binge eating in the evening.
Laboratory meal studies have provided the preponderance of the information about the pathophysiology of binge eating in BN. Typical meal studies involve patients and controls in a laboratory setting where either multiple-item or single-item meals are available and specific instructions are provided. These studies showed that when instructed to “let yourself go and binge” (with knowledge of access to a restroom for purging after the study) patients with BN ate larger meals than control subjects did and BN subjects rated the meals as typical of an actual binge (19–22).
An abnormally large meal is the result of decreased inhibitory controls of eating, increased excitatory controls of eating (23), or both. Early meal studies focused on possible decreased inhibitory controls of eating, i.e., decreased satiation during the meal. These studies identified candidate abnormalities in gastrointestinal and abdominal vagal afferent functions that are involved in satiation. Gastrointestinal abnormalities in BN include delayed gastric emptying (24, 25), reduction in gastric relaxation following food ingestion (26), diminished sensitivity to gastric distension (27), enlarged gastric capacity (25), and decreased release of cholecystokinin (24, 28, 29). Vagal afferent abnormalities in BN include alterations of somatic pain detection thresholds modulated by vagal afferents (30, 31), and normalization of pain thresholds and of bulimic symptoms following treatment with ondansetron, a serotonin type-3 receptor antagonist known to decrease vagal afferent activity (30, 32).
Each of these deficits is presumably a result of active eating-disordered symptoms, and at least some evidence suggests that certain abnormalities improve with treatment (e.g., tricyclic antidepressants improve postprandial cholecystokinin responses and satiety (28)). Each deficit could also conceivably contribute to further binge-eating and purging episodes. For example, delayed gastric emptying could be experienced as an uncomfortable sense of fullness even after a normal eating episode that would promote self-induced vomiting. The degree to which these deficits normalize with successful treatment of BN remains unknown.
A separate line of investigation supports the presence of increased excitatory controls of eating in people with BN. The excitatory controls are activated by orosensory food stimuli. Evidence for increased responsiveness to orosensory food stimuli includes:
1.
Individuals with BN have been found to continue to eat despite reporting maximal fullness (33). While this may be accounted for by a failure to experience maximal fullness as satiating or aversive, it may also be explained by increased responsiveness to orosensory stimulation.
2.
3.
Patients with BN increased the rate of food consumption as a meal progressed, in contrast to controls whose eating slowed as more food was consumed (36). This abnormal acceleration suggests an enhanced excitatory drive to eat, as rate of eating in animal feeding studies has been very closely linked with appetitive drive (37).
4.
5.
6.
Individuals with BN reported consumption of larger quantities of sweet nonnutritive products (e.g., chewing gum (43)) than non-eating-disordered controls, consistent with a heightened drive for, or heightened reinforcing value of, orosensory stimuli.
These observations suggest that the excitatory controls of eating are hyperresponsive in people with BN and are responsible, at least in part, for binge eating. Investigation of this possibility would be facilitated by an animal model. A review of the literature revealed that the SF rat was a good model of BN and purging.
1.2 SF Rat: An Animal Model of Bulimia Nervosa and Purging
As we have seen, intermittent binge eating and purging characterize BN. The combined effect of these two behaviors is to provide orosensory stimulation of palatable food, while minimizing its postingestive digestive, metabolic, and caloric consequences. This is similar to SF in animals. Reports of subjects with BN chewing and spitting out food and ingesting abnormally large quantities of nonnutritive products (e.g., chewing gum (43)) reinforces that impression (40–42).
The best current SF preparation is the chronic gastric fistula rat (44) (Fig. 1). When the screwcap of the fistula is closed, the ingested liquid food produces orosensory stimulation, is swallowed, accumulates in the stomach, empties into the small intestine, and is absorbed into the metabolic pathways. This is real feeding (RF). When the screwcap is open, ingesting the same liquid food produces the same orosensory stimulation, is swallowed, enters the stomach and immediately drains out of the stomach through the open cannula. Thus, drainage out of the cannula prevents all of the usual postingestive effects of the ingested liquid food. This is SF (See Sect. 2.1 for details of the surgical implantation of a gastric cannula and of SF tests).
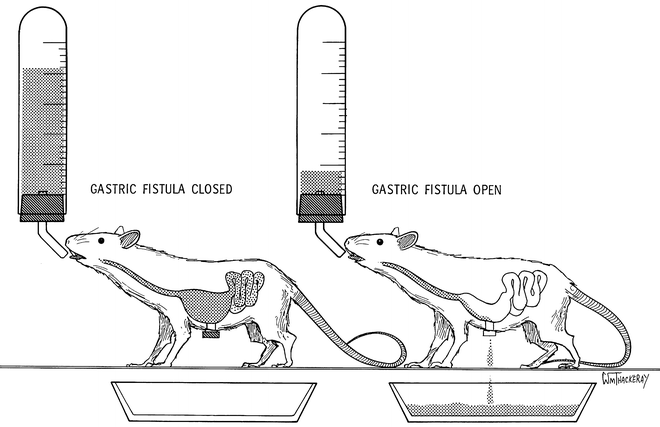

Fig. 1.
Chronic gastric fistula rat preparation for sham feeding. When the screwcap is removed from the cannula, ingested liquid drains out of the stomach (visual right ). This prevents postingestive negative feedback effects on intake from the stomach and small intestine. This is sham feeding. When the screwcap is in place and the cannula is closed, ingested food accumulates in the stomach and is emptied into the small intestine where digestion and absorption occurs (left ). This is real feeding. Reproduced, with permission, from (45).
For the purpose of animal modeling of binge eating, the critical fact about SF is that under a wide variety of deprivation and experiential conditions, and with many different liquid foods, rats eat larger meals when they are SF than when they are RF (46). In fact, when rats are given a palatable liquid food after overnight deprivation, they eat for hours with only short pauses between episodes of SF (47). This is binge eating in the rat.
When rats are deprived of food for 3 h (an interval within the range of the rat’s spontaneous intermeal intervals (IMI)), they SF a larger meal than they RF. Then they stop eating and display the sequence of behaviors that is the signature of postprandial satiety in the rat (48). However, they return to SF much sooner than after they RF. Thus, SF increases meal size and shortens the postprandial IMI. The shortening of the postprandial IMI probably models the more frequent reports of persistent urges to eat (34) and/or hunger (35) in the postmeal period in patients with BN than in controls.
These two effects have been analyzed in the rat into excitatory and inhibitory components. The larger size of the meal is accounted for by the excitatory effects of orosensory stimulation provided by SF and the lack of postingestive inhibitory negative feedbacks of hormones and visceral afferent nerves produced by draining the ingested liquid food out of the stomach. Thus, the larger meal is the result of orosensory excitation acting in the absence of postingestive inhibition. The only inhibition of eating that occurs in the SF rat is due to learned orosensory inhibition (49). It quickly extinguishes when repetitive episodes of SF occur ((50) and see below).
The shorter postprandial IMI is the result of a similar combination of orosensory stimulation and the loss of postingestive stimulation. The IMI after SF stops is about 50% shorter than after RF (51). This is produced by orosensory stimulation acting alone. That the IMI after RF is twice as long as after SF is due to the synergistic inhibitory actions of orosensory and postingestive stimuli for the control of the IMI. Note that the controls of meal size and of IMI are distinct.
The importance of the postingestive stimuli for the IMI has also been demonstrated in RF by draining the stomach of ingested food after eating has stopped (52). This models postprandial purging. When the gastric contents are drained, the IMI shortens. The abdominal vagus nerve is necessary for this effect because drainage of the stomach does not change the IMI in abdominal vagotomized rats. One of the strengths of the SF rat model of BN is that there is a wealth of information available, especially for the controls of meal size, about the peripheral and central mechanisms that are activated by orosensory excitatory stimuli and postingestive inhibitory stimuli (23). Description of these central and peripheral mechanisms, however, is beyond the scope of this chapter.
1.3 Modified SF in Women with BN and Purging
Modified SF (MSF) has been used to study orosensory control of autonomic, neuroendocrine, and metabolic mechanisms (cephalic reflexes). MSF excludes the postingestive effects of food by having subjects chew food and then spit it out without swallowing any (53–55). We modified MSF for the ingestion of liquid food (see Sect. 2.2). MSF had the following advantages over test meals in which the postingestive effects of ingested food occur: (1) MSF simulated the key features of the SF rat, in that the effects of excitatory orosensory controls of eating are measured, while the postingestive effects of food do not occur; (2) the sipping and spitting out of test solutions in the MSF technique is simple enough that all subjects learn it readily; (3) it is noninvasive and well tolerated by subjects; (4) MSF measures the effect of changing the palatability of liquid food (e.g., sweetness intensity) easily; and (5) various measures of ingesting can be quantified, such as amounts sipped and spit out, self-reports of sensory and hedonic aspects of the liquid ingested, rate of sipping, and intersip interval.
2 Methods
2.1 Methods for SF in the Rat
2.1.1 Materials
1.
Anesthetic: ketamine and xylazine or chloropent, a mixture of chloral hydrate and pentobarbital
2.
Anesthetic jelly
3.
General surgical instruments: toothed forceps, iris scissors, hemostats, wound clips, abdominal spreader, and scalpel with #10 blades
4.
Special surgical materials: gastric cannula with screw-cap, collecting tube connector, cannula bullet, cannula pliers, and Marlex mesh cut into 20 mm diameter circles. The cannula, bullet, pliers, and tube connector are not available commercially, but are easily fabricated in a machine shop. Contact gpsmith@med.cornell.edu for details. All instruments and materials must be sterilized by autoclaving
5.
Silk sutures 3-0 and 4-0
6.
Test cage with a slot cut through the middle of the floor from the front to the back. The cut must be wide enough to accommodate the collecting tube and to permit it to move freely as the rat moves around the cage during a test
2.1.2 Surgery
Preparation of the Animal and Cannula Placement
1.
Deprive the rat of food, but not water, overnight.
2.
Anesthetize the rat by intraperitoneal injection of an anesthetic that has a rapid onset of action and maintains a level of anesthesia for abdominal surgery for 1 h, e.g., ketamine (70 mg/kg) and xylazine (4.5 mg/kg) or chloropent (3 mL/kg).
3.
Shave the ventral surface of the rat from the costal margin to about 1 in. above the pelvis. Incise the midline with a #10 blade through the skin and the linea alba of the abdominal muscles. Make a small incision at the center of the midline incision and grasp the edges with toothed forceps. Insert the blunt blade of body scissors into the cavity beneath the midline. Use iris scissors to cut along the midline to expose the abdominal organs.
4.
Insert an abdominal spreader into the wound and open it against the sides of the wound. Leave it in place during the rest of the operation.
5.
Grasp the lower part of the stomach with toothed forceps. Put a 3-0 silk suture through the greater curvature of the stomach and clamp the free ends of the suture with a hemostat. Arrange the hemostat to provide sufficient downward tension on the stomach to keep it in position throughout the surgery.
6.
Note that the proximal part of the stomach is lighter in color than the distal part. The curved dividing line between these two parts is called the limiting ridge. For proper drainage of stomach contents, the cannula must be placed at the point where the limiting ridge meets the greater curvature of the stomach. Grasp the proximal part of the stomach just to the right (visual right) of the limiting ridge and cut a small (∼5 mm) hole through the wall of the stomach. While holding the left edge of the incision in toothed forceps, insert the circular base of the cannula with a clockwise twist using gentle pressure.
7.
When the base of the cannula is completely inside the stomach, insert gauze into the lumen of the cannula to prevent leakage of gastric contents. If leakage occurs, flush the peritoneal cavity immediately with isotonic saline (37 °C) to prevent peritoneal irritation by gastric acid.
8.
Place a purse-string suture (3-0 or 4-0 silk) through the wall of the stomach around the base of the cannula ∼5 mm from its shaft. Tie the free ends of the suture and invert the ends of the stomach wall into the stomach so that the outer (serosal) surface of the stomach fits tightly against the shaft of the cannula.
9.
Cut a small hole in the center of a Marlex mesh circle with iris scissors. Slide the mesh down over the cannula and spread the mesh out flat over the purse-string suture on the surface of the stomach. Tack the outer edge of the mesh to the stomach with 4-0 silk sutures that are spaced equidistant from one another.
Exteriorization of the Cannula Shaft
1.
Remove the spreader. Push the pointed end of the cannula bullet through the right abdominal wall at a point that holds the cannula in its present position when it is exteriorized.
2.
After the hole has been made through the abdominal wall, retract the bullet. Unscrew the pointed end of the bullet and expose the threaded end. Insert this end through the abdominal wall from the outside in.
3.
Grasp the shaft of the cannula with the cannula pliers, screw the threaded end of the bullet into the lumen of the cannula. When the cannula is firmly fixed to the bullet, remove the pliers and pull the shaft of the cannula through the bullet hole until resistance occurs due to the base of the cannula pressing against the peritoneal surface of the abdominal muscles. Hold the outer end of the cannula with the pliers and unscrew the bullet. Remove the gauze plug from the lumen of the cannula shaft and screw in the screwcap.
4.
Coat the area of skin around the base of the cannula shaft with anesthetic jelly. Screw down the nut that threads onto the shaft of the cannula until it is flat against the skin.
5.
Remove the spreaders and close the incision through the muscle layer with interrupted 3-0 silk sutures. Close the skin with wound clips.
Postoperative Care and Maintenance
1.
Return the rat to its home cage immediately after surgery. When the rat is completely awake, give it water to drink. About 6 h later, give it back its maintenance food.
2.
Remove the nut around the cannula shaft 48 h after surgery. Remove the wound clips from the midline incision 10 days after surgery. When the rat is fully recovered and with no signs of infection, testing can begin.
2.1.3 SF Test
1.
Remove the rat from its cage. Testing can be performed after some or no deprivation. Grip the cannula with cannula pliers and loop one finger-hole of the pliers over a finger of the hand holding the rat. With the free hand use a stubby screwdriver to unscrew the cap.
2.
Flush the stomach with isotonic saline (37 °C) by inserting a 20-mL syringe into the lumen of the cannula; inject the saline quickly and with slight pressure. Flush until no food particles are in the gastric drainage after two consecutive injections of saline.
3.
Attach the collecting tube by threading it into the cannula. Gently lower the rat into its cage while directing the collecting tube through the midline slot in the floor of the cage so that its tip hangs into a translucent plastic shoe box placed approximately 25 cm below the cage. Make sure that the tip of the tube stays in the box when the rat moves around the cage. Measure the volume of gastric drainage collected as well as the volume of liquid diet SF.
4.
Use three measures to evaluate the completeness of gastric drainage during SF of any liquid food. First, gastric drainage of ingested liquid must begin within the first 15 s of ingestion. Second, the total volume of gastric drainage must equal or exceed the volume ingested. Third, when the collecting tube is removed from the cannula at the end of a test, a significant volume of drainage does not occur. When these criteria are satisfied, gastric drainage is complete. If all three criteria are not met, drainage is incomplete and the results of that test must be discarded. If this occurs repeatedly, remove the rat from the experiment.
5.
At the end of the test, replace the screwcap and return the rat to its cage. Restore maintenance food and water.
2.2 Modified SF in Humans
2.2.1 Materials
Solutions
Test solutions are prepared fresh 18–24 h prior to the experimental day, using aspartame in concentrations of 0, 0.145, 0.3, 0.75, and 2.8 g/L, or 0, 0.01, 0.03, 0.08, and 0.28% wt/wt, respectively. Cherry-flavored Kool-Aid® (1.902 g/L) is added to make the solutions more palatable and more comparable to beverages commonly consumed in the United States. Each sip container contains 1,900 mL of the test solution (2 L are prepared with 100 mL drawn off for the taste-test samples).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


