Chapter 6 SEMEN EVALUATION
The general aim of semen evaluation is to assess the prospects of fertility of individual stallions or individual semen samples such as ejaculates or doses of fresh, cooled-transported, frozen/thawed semen).1–10
The target of semen evaluation is to clarify if quantitative and qualitative spermatological parameters are in compliance with the minimum requirements fixed for biological composition of stallion semen. Minimum standards are empirically determined averages with consideration of the standard deviation of spermatological parameters in a fertile stallion population. To define standards, variation, and minimal criteria of stallion semen, Klug11 and Parlevliet et al.12 in the Warmblood, Dowsett and Pattie13 and Pickett et al.14 in Quarter Horses and Thoroughbreds, and Paccamonti et al.15 in Miniature stallions reported semen parameters such as volume with and without gel, sperm concentration per cc, total number of spermatozoa per ejaculate, percentage of motile spermatozoa, percentage of morphological normal spermatozoa, and seminal pH. Recommendations regarding semen standards used in artificial insemination are published as “Minimum standard requirements for stallion semen for AI” by the World Breeding Federation for Sport Horses (WBFSH) (Fig. 6-1).

Figure 6-1 Recommendations for commercial stallion semen standards used in AI practice published as “Minimum standard requirements for stallion semen for AI” by the World Breeding Federation for Sport Horses (WBFSH). Available at http://www.wbfsh.org/?GB/Activities/Semen%20standards.aspx.
The predictive value of semen analysis in the evaluation of stallion fertility has been reported by numerous investigators.16–24 The failure to find strong correlations between fertility and quantitative assessments using either standard spermatological parameters or the more sophisticated tests has been reported in several domestic species25,26 and in the horse.27–29 The fact that the relationship between sperm quality determined by different assays and stallion fertility is often questionable and might be explained by the use of the limited numbers of examined males and females and the use of inconsistent fertility scores in the studies.30,31
Specific indications for semen evaluation exist in AI practice, such as breeding soundness evaluation of stallions, preseasonal and seasonal monitoring of stallion semen quality in AI centers in case of liability claims due to sperm quality, and evaluation of extenders and/or material toxicity tests, or research.32–35
Parameters usually included in a conventional evaluation of raw semen quality are either quantitative (e.g., volume gel, gel-free, and total semen volume; sperm concentration per ml; total number of spermatozoa in the ejaculate) or qualitative (e.g., percentages of motile spermatozoa, sperm morphology,16,18,36–39 longevity of sperm motility after cooled storage,40 and bacteriological status). Composition of seminal plasma, although not routine, can be performed in cases where stallions have unexplained low motility or longevity.41–43
Although these evaluations provide abundant information, it is well accepted that these parameters give only crude information on the fertility of stallions. Prediction of male fertility could be improved if additional parameters based on functional characteristics of spermatozoa are considered.34 Therefore, several functional tests have been developed and are summarized in this chapter together with established routine tests for analysis of stallion spermatozoa. In commercial AI settings, however, a quick assessment of appearance, volume, concentration, and motility remains the most common evaluation.
ROUTINE MACROSCOPIC EVALUATION (GROSS APPEARANCE) OF STALLION SEMEN
Volume of the ejaculate is dependent on species, breed, age, and environment. Environmental factors affecting seminal volume may include feeding, housing, teasing, soundness, method and frequency of semen collection (collection of total ejaculates or seminal fractions44), and time of the year (e.g., small volume during the non-breeding season).45 In general, species with intrauterine ejaculation (e.g., stallion, boar) release larger ejaculates than species with vaginal ejaculation (e.g., cattle, sheep). Seminal volume is largely influenced by the amount of secretions resp. seminal plasma of the accessory sex glands. The seminal volume should be determined in terms of gel-free volume representing main parts of the sperm-rich fraction of a stallion ejaculate; thus, sperm-free pre-ejaculatory fluid secreted from urethral and bulbourethral glands as well as the stallion-specific gel fraction released by the seminal vesicle glands in the second sperm-poor fraction should be recorded separately. The volume of the semen sample should be measured in a pre-warmed, sterile measuring cylinder. The total volume of the ejaculate should be 60–120 ml and the gel-free volume 30–100 ml. These figures are highly variable between stallions36 and with season45 and collection frequency.16,18,44 Reduced seminal volume can be a result of failure of emission of semen (e.g., ejaculatory failure syndrome, obstruction of deferent ducts).34,46 Correct measurement of seminal volume in combination with sperm concentration is a prerequisite for calculation of total sperm count of the ejaculate, with the latter being the most important measure determining the amount of producible AI doses.
MICROSCOPIC AND FLOW CYTOMETRIC EVALUATION OF STALLION SEMEN
Sperm Concentration
Traditionally sperm concentration is determined by means of cell-counting chambers (hemacytometer method), upon recommendations of the World Health Organization. The Neubauer chamber or hemacytometer is regarded as the golden standard for evaluation of sperm concentration.47,48 Defined aliquots of the sperm sample are dissolved and immobilized in 10% formol/NaCl solution. After gentle stirring of the sample, the hemacytometer chambers are loaded. According to sedimentation, sperm are counted microscopically in a total of 10 squares out of two chamberfields. Contingent upon sperm sample volume, fixed variables for height, and volume of the hemacytometer chamber, the resulting sperm concentration can be calculated. The accuracy of the procedure depends significantly on precision of pipetting, careful stirring, and absolute number of counted sperm (at least 100).49 Considering these rules, the hemacytometer method is regarded as a comparably precise method to measure concentration of spermatozoa. However, the amount of time required is disadvantageous.50 Therefore, this method is often not applied in routine laboratory practice.
Additional possibilities arise out of integration of computer-assisted sperm analysis (CASA) (sperm motility analysis) into laboratory practice; these machines can determine sperm concentration and sperm motility simultaneously with adequate accuracy. The use of a flow cytometer in combination with fluorescence dye–marked sperm has been reported as a valuable tool to determine sperm concentration with high precision.51 Because of methodical and device-related reasons, flow cytometry is expensive. Due to the complexity of sperm preparation, as well as the instrument itself and its cost, it finds application in specialized labs.
Recently, easy-to-use counting equipment has been developed; the NucleoCounter SP-100 (ChemoMetec, Alleroed, Denmark) counts mammalian cell nuclei stained with the DNA-specific fluorescent dye, propidium iodide. Propidium iodide is excluded from viable cells. This is used in the NucleoCounter to estimate the concentration of non-viable cells and the concentration of total cells in a suspension.52
Sperm Motility Analysis
Light Microscopy
The motility of gel-free semen should be estimated immediately after collection on a pre-warmed microscope slide. Prerequisites are a phase-contrast microscope with a warm surface (37°–40°C). The typical procedure to assess sperm motility is done by placing approximately 2-5–μl droplets on a pre-warmed slide covered with a warm cover-slip and using a phase-contrast microscope at a magnification of 150–200×.4 Motility is expressed, in percentage form, as oscillatory or progressive (i.e., those that are alive but are moving in a circle around their own axis and those that are actively moving forward). Sperm from a normal fertile stallion should have an immediate progressive motility ≥60%. Abaxial attachment of the midpiece to the sperm head is physiological in stallion spermatozoa and responsible for a circular motion of specific aliquot of stallion semen samples. Therefore, estimating only the progressive motility may underestimate good motility from some stallions. Preferably, semen should be extended before it is analyzed because raw stallion semen tends to agglutinate and individual sperm movement could be difficult.4 Visual estimation is inexpensive, simple, and accurate if analysis is performed by an experienced technician. Nevertheless, the microscopic evaluation is a subjective procedure that requires experience and a regular control and training of the laboratory personnel. Standardization is indicated and recommendations include incubation of the sample at 37°C for 2 minutes in a waterbath, sperm concentration of 25–50 × 106 sp/ml, and chamber 10–20 μl; a minimum of two drops from the suspension should be analyzed. Evaluation of at least four or five fields near the center of the coverslip is recommended.4,46 Motility at the edges declines more rapidly than in the center as a result of drying and exposure to air. If subjective motility is used experimentally, it should be done blindly, preferably by averaging values of two evaluators. Simultaneously with the evaluation of sperm motility, agglutination incidence of spermatozoa, and number of foreign cells should be recorded.1,2,24,38,39
Occasionally cylindrical “round” cells are present in semen. They represent early undifferentiated stages of spermatogenesis. These round cells are tail-less and up to eight times larger than the sperm head. Immature spermatogenic cells vary in size and typically possess round, dark-staining nuclei. This is in contrast to the homogeneous size of white blood cells and the distinctive nuclear characteristics of the neutrophil. These immature “round” spermatogenic cells may appear in the ejaculate in increasing numbers when stallions are affected with early stages of testicular degeneration.38,39
Computer-Assisted Sperm Analysis (CASA)
Classical microscopic assessment has the disadvantage that sperm motility estimates can vary among examiners. Computer-assisted sperm analysis (CASA) should offer a more reliable and repeatable means of assessing sperm parameters such as motility.53
CASA allows for the objective determination of a variety of motility parameters, sperm concentration, and newest models also have a morphology module, although limited to sperm head morphology. 54
In 1985 the first commercial CASA system developed specifically for evaluation of sperm motion was called the CellSoft system. The second commercial system developed specifically for evaluation of sperm motion was the Hamilton Thorn Motility Analyzer, HTM-2000, introduced in 1986. The impetus for development of this system was quantification of changes in stallion sperm during storage in the Equitainer. This system had technical advances including an integrated optical system, video display, warming tray, and an automated positioning of the sample to pre-determined locations. The near-infrared illumination and dark-field optics of the initial system soon were replaced in the HTM-S by visible-light illumination and phase-contrast optics.53 Today several other systems are in the market, such as the Sperm Vision from MiniTube. Video images for computerized sperm motion analysis are obtained from viewing fields of motile sperm using a microscope. A set number, usually 20 to 30 successive video frames were analyzed at a constant rate, typically 30–60 frames per second (Fig. 6-2). When all frames for a given field have been recorded, image-processing computer software detects and tabulates algorithms and distinguishes sperm from non-sperm objects and reconstructs sperm tracks.
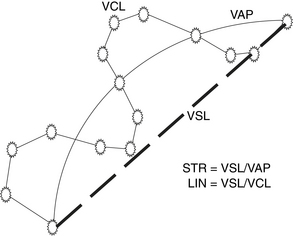
This technique is objective and evaluates the motility according to the given criteria.55–57 It allows analysis of more specific characteristics of spermatozoa. Kinematics as shown in Figure 6-3 are percentages of total motility (MOT) and progressive motility (PMOT), as well as mean straight-line velocity (VSL), mean curvilinear velocity (VCL), average path velocity (VAP), straightness (STR = VSL/VAP), linearity (LN = VSL/VCL), lateral head displacement (LHD). Due to the high cost of the instrument, computerized sperm image analysis systems are used primarily in well equipped AI centers, commercial AI organizations, or research facilities. Today, more complex CASA instruments that would overcome the limitations of current instruments, such as being able to simultaneously analyze sperm motion characteristics, sperm viability, and morphology, are in development.47,52,58 On the other hand, development of any device might be inhibited by perception of an unfavorable cost–benefit ratio.
Each CASA system needs standardization and validation concerning calibration and technical settings (e.g., frame rate, light settings, recognition of spermatozoa by sperm head size and brightness, tail detection, recognition of immotile particles) to provide accurate and repeatable results.53,57 Potential sources of error should be minimized to allow comparison of data obtained by different systems and groups.7 However, no international standardization in equipment settings has yet been implemented, which does not allow direct comparison of results between laboratories. Thus, there is an urgent need for users of CASA to agree on standard analysis parameters.7,53 In addition to having correct settings, repeatability and accuracy of the data within a sample and the relationship between CASA and subjective evaluation of motility by an experienced operator are important factors that must be considered to ensure that the data are accurate. Using an HTM analyzer, Bataille et al.59 evaluated samples from 62 stallion ejaculates that had been frozen. Their results indicated that the variability within a sample was only 2% in the percentage of rapid sperm (3.5 μm/sec in VAP), and 2 million in sperm concentration when at least 12 measures were realized. The analysis was done on two straws per ejaculate, with two drops per straw and three fields per drop. These authors reported a significant correlation between linearity, mean path velocity, percentage of rapid spermatozoa, and motility analysis determined subjectively with light microscopy. Kirk et al.28 reported that the mean percentages of motile sperm in cryopreserved stallion semen samples were 26% and 36% when the same samples were evaluated visually and using CASA, respectively. Interestingly, the inter-assay variability was 16% for both tests, and the intra-assay variability was 5% and 10%, respectively, for the two assay methods.
The main factors that affect the CASA results are the type of chamber used for the evaluation and the amount of debris or foreign material in the sample. Most CASA systems use Makler chambers, but semen dries quickly at 37°C; however, newer CASA models are faster and can analyze 400 cells in 2 minutes. Varner et al.54 demonstrated that field within chamber accounted for most of the variability in the CASA analysis and recommended that three chambers per ejaculate and three fields per chamber be evaluated, which would result in the analysis of approximately 500 cells per sample. The SpermVision system analyzes motility of semen samples with the help of a standardized ready-to-use Leja Chamber (Leja Products, Nieuw-Vennep, The Netherlands). The ideal sperm concentration is between 25 and 50 ×106 sperm/ml. However, time between filling of the chamber and starting the measurements, the duration of the analysis, and selection of and number of measuring positions in the chamber are the most important technical factors to consider.
In addition, the type of extender and ejaculate characteristics are important factors to consider. Sperm agglutination and the presence of non-sperm particles such as egg yolk are factors that can affect the analysis and results. To overcome this problem, many laboratories use (1) clarified egg yolk suspensions obtained by centrifugation,60 (2) detergents in the extender, such as Equex STM (delta-amino-sodium lauryl sulphate) to solubilize the egg yolk lipids and lipoproteins, (3) non-fat dry milk glucose extender to dilute frozen-thawed semen samples before CASA evaluation, and (4) fluorescence dyes that do not affect motility (e.g., Hoechst 33342) to differentiate sperm cells from egg yolk particles in CASA systems equipped with epifluorescent illumination (e.g., HTM, SpermVision).47,58
Motility had a low (0.45) but significant correlation with the first-cycle pregnancy rate of 177 mares inseminated with frozen semen from nine stallions.61 However, predominant motility of the spermatozoa is poorly correlated with fertility.18,23,56,62 This seems reasonable because motility is only one attribute of the sperm. Thus, attempts have been made to evaluate several sperm attributes as a means of determining semen quality58,63–65 or to differentiate between subpopulations of sperm with different motility characteristics.66 Kirk et al.28 compared flow-cytometric assays for viability, acrosome status, and mitochondrial membrane potential of frozen-thawed sperm with visual and computer-assisted motility analysis; the results of these assays were correlated with stallion sperm fertility. The ultimate goal of multi-parametric sperm analysis is to be able to distinguish sperm samples that could have good fertilizing potential from those likely to have poor fertility.
Longevity of Spermatozoa
Cooled-stored or frozen-thawed semen samples may be examined on warm microscope slides after different time intervals and under different storage conditions (+5°C, 37°C test of thermoresistancy). Motility estimations under different conditions for frozen-thawed spermatozoa have been proposed: Frozen-thawed sperm have been evaluated at1°C, for several days until no motile cells are present67; at 4°C during 5 days,68 at 38°C for 3 hours.69 Longevity of motility is used in 15 centers out of 21 as reported in a survey conducted by Samper and Morris,70 while four centers incubated sperm at 38°C for 4 hours. Nine centers evaluated semen by incubating at 20°C during 12–48 hours. Acceptable motility thresholds varied among laboratories ranging between 5% and 20%. Today most laboratories would incubate frozen-thawed sperm at 38°C for 2 hours, expecting a minimum of 15% motility after that period. Although visual motility and the straightness of sperm motility conducted 90 minutes after thawing were correlated with seasonal fertility (0.56 and 0.55, respectively), data from no single assay were significantly correlated with first-cycle fertility rates.28
Post-Thaw Motility of Stallion Spermatozoa
Samper and Morris70 reported in their survey of equine AI centers freezing semen that CASA is the basic method for all the centers. Before freezing, 13 of 21 centers required >50% motile sperm, while 8 of 21 required >60% before discarding the semen. After freezing and thawing, the threshold is quite different between centers: 2 centers required a minimum of 25%, 10 centers 30%, 8 centers 35%, and 1 center 40%. Even though motility estimation before freezing is well correlated with motility after freezing and thawing, it cannot be considered as a test to predict poor freezable ejaculates. Vidament71 summarized 20 years of field results with frozen semen in France and reported that stallions and ejaculates were selected on the basis of CASA measured post-thaw motility; an ejaculate was considered acceptable if motility was ≥35% rapid sperm. In their study a rapid sperm was defined as a motile cell with an average path velocity between 30 and 40 μm/sec.
In a previous study Loomis72 reported that acceptable ejaculate frozen following the Select Breeders Services freezing protocol was one that yielded post-thaw progressive motility of ≥35% and ≥25% following a 30-minute incubation at 37°C.
In a recent study, Loomis and Graham73 reported that following incubation, the motility of the extended semen as determined using CASA. A minimum of 400 motile or 1000 total sperm from more than five fields were analyzed for motion characteristics at 60 frames/sec for 40 frames. The stage of the microscope was maintained at 37°C throughout analysis. For this program, a sperm is considered progressively motile if it has a VAP >50 μm/sec and STR value >75%. A sperm with VAP <20 μm/sec is considered non-motile in the calculation of percentage of motile spermatozoa.
Computer-Assisted Sperm Morphometry
Morphometric analysis consists of measuring the length, width, area, and perimeter of the sperm head performed on stained smears by computer-assisted imaging. Morphometric analysis of the sperm head by computer-assisted microscopy is preferably used in specialized laboratories mostly using Feulgen or hematoxylin stains.74–77 Software is available by using either the ASMA (automated sperm morphology analysis, Hamilton Thorne Research, Beverly, MA) or SAMBA-TM2005 software (Alcatel TITN, Meylan, France). Several reports have indicated a positive and significant correlation between sperm head size and stallion fertility.5,78,79
Sperm Morphology Analysis
A major part of any breeding soundness examination is an evaluation of sperm morphology. It requires specialized equipment (a light microscope equipped with a planapochromatic ×100 oil immersion objective and a condense enabling bright field and differential interference contrast microscopy), expertise and experience of the technician, and often a great deal of time and patience. A minimum of >100 cells per slide should be analyzed and classified.2,4,8,37–39,80,81
Many different staining techniques have been devised for examining sperm morphology in stallion.38,39 Sperm morphology can be evaluated by examining stained smears under bright field microscopy. Stains that are routinely used are: Williams,82 Karras,83 Eosin Anilin Blue,84 nigrosin-eosin,85 Spermac,86 and Feulgen87). Nigrosin-eosin stain85 is commonly used because it is effective and simple, spermatozoa are readily visualized, and it is a “live-dead” stain, allowing the practitioner to assess membrane integrity at the same time as morphology. The nigrosin-eosin stain produces a dark background on which the sperm stand out as lightly colored objects. Normal live sperm exclude the eosin stain and appear white, whereas “dead” sperm (i.e., those with loss of membrane integrity) take up eosin and appear pinkish in color. A number of variations of the nigrosin-eosin stain exist.87 The nigrosin-eosin formulation is prepared by dissolving 5% w/v nigrosin, 0.6% w/v eosin, 3% w/v sodium citrate-dihydrate in water. The pH of the stain is then adjusted to 7.0 and passed through a filter paper.
Furthermore, a technique preferred in many laboratories for evaluating sperm morphology is to visualize sperm under phase-contrast microscopy or preferably under differential interference contrast microscopy. This method allows excellent visualization of sperm defects, but the requirement of 100× objective and excellent microscope optics limits its use to well-equipped laboratories. When one of these methods is used, it is important to allow some time after the preparation of the wet mount for sperm to settle flat on the slide. By running this “wet-mount” technique, the sperm are first fixed (~1:4 ratio) with glutaraldehyde or buffered formol saline88–90 and can be stored for prolonged periods in that solution. This procedure is particularly useful for assessing acrosomal integrity. In addition, incidence of artifacts is lower compared with stained smears.
It seems obvious that conventional light microscopic evaluation of sperm alone does not fully provide potential indicators of functional impairment in spermatozoal organelles. Thus, Veeramachaneni et al.38 reported a technique that combined evaluation of sperm under a wet mount and a dry smear stained with toluidine blue for bright field microscopy. Transmission electron microscopy using thin sections stained with uranyl acetate and lead citrate have also been reported. This technique provides information of the ultrastructural morphology of sperm organelles and has proven to be a useful tool to help in the diagnosis of infertility of stallions.34,91
Two types of classifications are commonly used. Abnormalities can be classified with regard to the anatomic site of the defect; thus, the head, midpiece, or tail is affected, with some sperm having defects in more than one site.22,92 The other system classifies defects into primary (failure of spermatogenesis), secondary (failure of maturation), and tertiary (damage occurring during or after ejaculation) categories or abnormalities.93 Generally, primary defects are the more severe and are thought to originate during spermatogenesis within the seminiferous epithelium of the testis. Disturbances in spermatogenesis are best detected by evaluating sperm morphology; with this in mind, sperm morphology represents one of the most important sperm assays because of its moderate-to-high correlation with fertility in the stallion. Secondary defects are regarded as less serious and thought to arise during epididymal passage and storage. Tertiary defects could be iatrogenic and may occur by mishandling of sperm after ejaculation. Limitations of this classification system are the unknown origin of some sperm defects and the fact that primary defects are not necessarily more deleterious to fertility than secondary defects, a common misinterpretation of this system. Therefore, the utility or physiological basis of this classification scheme is controversial in the literature.
The Society for Theriogenology sperm morphology evaluation form, also called the differential spermiogram,94,95 records the following sperm categories: normal sperm, abnormal acrosomal regions/heads, detached head, proximal droplets, distal droplets, abnormal midpieces, and bent/coiled tails. The presence of teratoid sperm and of other cells (round germ cells, white blood cells, red blood cells, etc.) should also be indicated. Acrosome defects include knobbed, roughed, and detached acrosomes. Head defects include microcephalic (small, underdeveloped, or dwarf), macrocephalic (large or giant), pyriform (narrow at the base), tapered (narrow), nuclear vacuoles (pouches or craters), and multiple heads. Midpiece defects include midpiece reflex (simple bent or folded midpiece), segmental aplasia of the mitochondrial sheath, fractured, swollen (thick, pseudodroplet), roughed (corkscrew), swollen/roughed/broken (Dag-like), disrupted sheet (filamentous), duplicated, and stump tail. Bent or coiled tails refer to those sperm in which both the midpiece and the principal piece are bent or coiled, or the distal part of the principal piece is coiled. The percentage of normal and abnormal detached heads (tailless or separated heads) could be recorded separately.
The classification of sperm defects as major and minor has been accepted by some authors,96,97 suggesting that major defects cause early embryonic death or prevent fertilization (e.g., acrosomal defects preventing zona binding and subsequent fertilization of the egg, nuclear defects may result in EED or nonfertilization) and minor defects (e.g., abnormalities of the sperm tail) alter sperm motility, so the spermatozoon cannot reach the egg. Some abnormalities are compensable and some are non-compensable.98 A non-compensable abnormality gives the animal a poor fertility prognosis.
The current method of classification is to record the numbers of specific morphologic defects. However, when more than one abnormality is found on a single spermatozoon, it is an indication of a more severe disturbance of spermatogenesis and possibly a poorer fertility prognosis.99 This procedure is considered superior to the traditional system because it offers specific information with regard to the semen sample analyzed and avoids erroneous speculations about the origin of these defects. It has been generally accepted that the total number of morphologically normal sperm in ejaculates may provide more information regarding the fertility of a stallion than the percentage or absolute number of morphologically abnormal spermatozoa.
Fluorophores and Flow Cytometry
Many different fluorescent stainings and combinations of fluorophores have been developed to evaluate different sperm characteristics in several mammalian species (see Morrell,100 Gadella et al.,101 and Caiza de la Cueva et al.102 for review). Fluorescent sperm can be analyzed by fluorescence microscopy or by flow cytometry: proportions of live and dead cells,103–107 mitochondrial function,108–110 acrosomal integrity,111–119 capacitation status,120–122 intracellular calcium concentration of spermatozoa,123 and sperm chromatin or DNA content124–126 have all been evaluated. Although most fluorescent stains can be used in combination with fluorescence microscopy, flow cytometry has become the most used method. Basically, a flow cytometer has a light source, usually a laser or a mercury arc lamp, a sample chamber, flow cell or jet-in-air nozzle, sheath-fluid stream, photodetector or photomultiplier tubes that convert the collected light to electronic signals, a signal-processing system that processes digital signals from analog output, as well as a computer to direct operations, store the collected signals, display data, and drive the sorting process. This in itself provides a method of rapidly analyzing a population of cells into subpopulations and determining any changes relative to the percentage of each.127 There are several types of lasers available, which differ in the gain medium that is used to amplify light (e.g., argon ion [365, 488, or 514 nm], krypton [567 or 646 nm] and helium-neon [633 nm], mercury arc lamp lasers), specific wavelength emitting solid state laser). Spermatozoa are labeled with a fluorochrome of choice and used in either the viable or fixed state. The choice of fluorochrome is influenced both by the application and the excitation wavelengths available on the flow cytometer.100,128 Once labeled, spermatozoa are aspirated and made to flow rapidly through the flow cell where they are illuminated by the laser beam; scattered and emitted light is collected by a typical arrangement of lenses, optics, and filters, resulting in the measurement of specific bands of fluorescence. Flow cytometry permits the observation of physical characteristics, such as cell size assessed by the so-called forward scatter, the granularity of cells differentiated by side scatter, and any fluorescence signal emitted by fluorophore-stained spermatozoa that can be collected with photomultiplier tubes. Flow cytometry also has the capacity to detect labeling by multiple fluorochromes associated with individual spermatozoa, meaning that more than one sperm attribute can be assessed simultaneously, which offers the advantage of a more accurate fertility prediction because several sperm attributes can be tested simultaneously. A further advantage of flow cytometric assessment is that large numbers of spermatozoa can be analyzed by high flow rates of 200–800 s−1 in a very short period. Routinely a total of 10,000 spermatozoa are analyzed, which is substantially more than the total of 100–200 cells generally observed by microscopic analysis. Thus, flow cytometry is a very rapid and sensitive method for the detection of subtle differences among thousands of spermatozoa that may not be apparent using other techniques. Multiple aspects of sperm function can be assayed simultaneously. The disadvantages of using the flow cytometer for semen analysis are the costs, the need of a skilled operator, and, because of the size and sensitivity of the apparatus, the requirement of a dedicated position in the laboratory.
Sperm Plasma Membrane Integrity and Sperm Viability Assays
The plasma membrane of sperm is composed of three different compartments or regions: the outer acrosomal membrane, the post-acrosomal region of the sperm head, and the middle and principal pieces of the sperm. Thus, to analyze the integrity of these different plasma membrane compartments, classical stains such as eosin-nigrosin85 and eosin-aniline-blue84 or fluorophores are used. Fluorescent stains such as propidium iodide, ethidium bromide, diamidino-2-phenylindole [DAPI] or bisbenzimide–Hoechst 33258 bind to and stain the DNA of sperm possessing defects of post-acrosomal plasma membrane of the sperm head. Whereas the integrity of the plasma membrane covering the principal pieces of sperm can be assessed by analysis of sperm motility or the hypo-osmotic swelling test (HOST), the integrity of the plasma membrane covering the acrosome is focused on the integrity of the outer acrosomal membrane using microscopy (DIC), fluorometers, and flow cytometry.104,129
Fluorescent staining of spermatozoa to determine viability can be approached in two ways: fluorochromes used to indicate viable (intact plasma membrane) cells and those used to indicate non-viable (damaged plasma membrane) cells. Use of the flow cytometer to determine the proportion of viable spermatozoa can be achieved by the use of fluorochromes (e.g., 6-carboxyfluorescein diacetate [CFDA], calcein AM),105,117,130,131 which penetrate the plasma membrane and are de-esterified by esterases in viable spermatozoa to non-permeant fluorescent compound that is retained in the cytoplasm, causing them to fluoresce green when exposed to the appropriate wavelength of light. Therefore, only live cells with both active intracellular esterases and intact plasma membranes will fluoresce with these markers. Plasma membrane permeant DNA fluorochromes (e.g., SYBR-14 excitation/emission 488/515 nm wavelength65,106) label nuclei of viable cells. SYBR-14 is cell permeable and brightly stains only the nuclei of living cells, emitting green fluorescence in response to excitation. Nucleic acid stains are less variable than enzyme-based stains, and sperm DNA is a preferable target because of its stainability and staining uniformity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree