Selection criterion (High vs. low vulnerability)
Drug-related behavior
Drug/reinforcer
Reference
Novel environment reactivity(HR vs. LR)
Drug-induced locomotor activity (HR > LR)
Amphetamine
(27)
Morphine
(28)
Self-administration (HR > LR)
Amphetamine
(27)
Impulsivity: delay discounting (HiI vs. LoI)
Acquisition (HiI > LoI)
Cocaine
(33)
Acquisition (HiI > LoI)
Ethanol
(34)
Escalation (HiI > LoI)
Cocaine
(35)
Reinstatement (HiI > LoI)
Cocaine
Impulsivity: 5CSRTT (HiI vs. LoI)
Acquisition (HiI > LoI)
Cocaine
(37)
Self-administration (HiI > LoI)
Cocaine
(37)
Reinstatement
Cocaine
(29)
Incentive salience (ST vs. GT)
Self-administration (ST > GT)
Cocaine
(31)
Reinstatement (ST > GT)
Cocaine
(31)
Locomotor sensitization
Cocaine
(38)
Impulsivity (5-CSRTT; GT > ST)
Food
Wheel running (HiR vs. LoR)
Self-administration (HiR > LoR)
Cocaine
(32)
Reinstatement (HiR > LoR)
Cocaine
(32)
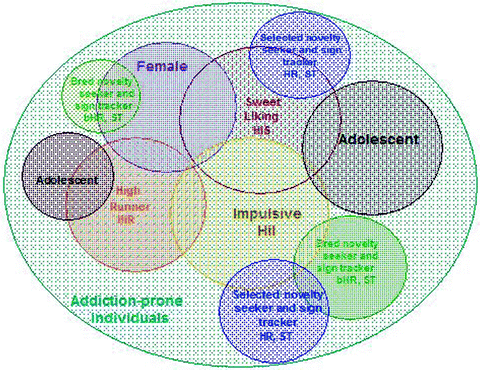
Fig. 1.
Hypothetical overlap of addiction vulnerability factors that apply to food, exercise, or drug rewards.
2.9.2 Selective Breeding for Traits Associated with Drug Abuse
Another approach to investigating genetically mediated differences in addiction vulnerability involves breeding rodents based on bidirectional behavioral criteria, as summarized in Table 2. By mating animals that exhibit extremely high or low measures, it can be shown that the behavior of interest is under genetic influence, because successive generations show more stable and robust corresponding behaviors. A prominent example of this procedure is found in an alcohol study in which breeding for differential drug intake was conducted by selecting animals on measures of high and low ethanol intake (52). Subsequently, multiple rodent strains have been selectively bred based on varied behavioral and physiological responses to alcohol in humans (53, 54). Such criteria have included alcohol consumption (55, 56), sensitivity to withdrawal (57), ethanol-induced hypothermia (58), and locomotor reactivity (59).
Table 2
Selectively-bred rats that differ on drug- and food-motivated behavior
Selective-breeding criterion (High vs. Low vulnerability) | Drug-related behavior | Drug/Reinforcer | Reference |
|---|---|---|---|
Ethanol consumption (HAC vs. LAC) | Impulsivity (delay discounting; HAC > LAC) | Saccharin | (41) |
Impulsivity (probabilistic discounting; HAC > LAC) | Sucrose | (42) | |
Impulsivity (response disinhibition; HAC > LAC) | Sucrose | (43) | |
Exercise (HAC > LAC) | Wheel-running | (44) | |
Novel environment reactivity (HR vs. LR) | Acquisition (HR > LR) | Cocaine | (11) |
Active-avoidance learning (RHA vs. RLA) | Acquisition (RHA > RLA) | Cocaine | (45) |
Locomotor Sensitization (RHA > RLA) | Cocaine | (46) | |
Impulsivity (delay discounting; RHA > RLA) | Food | (40) | |
Impulsivity (response disinhibition; RHA > RLA) | Food | (40) | |
Novel environment reactivity (RHA > RLA) | — | (46) | |
Lewis vs. Fischer 344 | Acquisition | Cocaine | (47) |
Self-administration | Cocaine | (48) | |
Ethanol | (49) | ||
Locomotor sensitization | Cocaine | (50) | |
Reinstatement | Cocaine | (51) |
The selective breeding approach was also applied to other drugs of abuse, such as diazepam (60), opiates (61), methamphetamine (62, 63), nicotine (64), and cocaine (65). Similar to studies that selected outbred animals for behaviors directly or indirectly related to the behavioral effects of drugs, animals selectively bred for high and low addiction vulnerability also showed related behavioral features in measures indirectly related to drug abuse vulnerability. For example, animals bred for high (vs. low) alcohol consumption displayed greater impulsivity using delay- and probabilistic-discounting procedures (41, 43), as well as tasks of impaired response inhibition (43). High (vs. low) alcohol-consuming animals also showed greater avidity for natural rewards, such as exercise (44).
In addition to selective breeding for intake of a substance (e.g., alcohol intake), the procedure of selectively breeding for high and low phenotypes has been applied to behaviors that are related to drug addiction (Table 2). For example, one criterion is novelty-induced locomotor activity, the same measure shown to have a positive relationship with response to amphetamine by Piazza et al. (27) in outbred rats. Rats selectively bred for high activity in this paradigm acquired cocaine self-administration more rapidly than those bred for low activity (11). The two behaviors that have received the most attention are binging on food (food addiction) (5, 66, 67) and impulsivity for food (see reviews by (37, 68, 69)). The fundamental principle unifying the literature described so far is the following: addiction vulnerability and certain types of behaviors reliably covary; therefore, it is probable that they are mediated by common underlying mechanisms. High or low avidity for sweetened dietary substances is another common feature to many of these models, and its interaction with addiction vulnerability is the focus of the remainder of this chapter.
In some cases the traits that predict drug abuse are bidirectional; for example, many of the animals screened or selectively bred to show divergence in drug seeking or drug response also show divergence in avidity for sweet substances. For instance, the selected HR rats respond more robustly to sucrose (70) compared to LR rats, while rats selected for HiI using the 5-CSRTT show more operant responding to sucrose compared to LoI rats (71). Further, Roman high avoidance (RHA) rats selectively bred for high rates of active avoidance learning consume more sweetened dietary substances, such as a saccharin solution, compared to those selectively bred for low rates of avoidance learning (RLA) (72, 73).
In many cases, behaviors that are selected for or selectively bred are interchangeable or substitutable behaviors related to addictive behavior. For example, rodents selected from outbred stocks or by selective breeding for high and low alcohol consumption ingest high and low amounts of sucrose and saccharin solutions, respectively (74–77). Conversely, rats screened for high consumption of sweet substances ingest more ethanol (78), amphetamine (79), and morphine (80) than rats screened for low ethanol intake. In fact, Table 3 shows that rats selected for an affinity for a variety of substances or events have elevated drug-seeking behavior compared to their counterparts with low selection criteria.
Table 3
Selected and selectively-bred individual differences for sweet preference and their effects on drug- and food-motivated behavior
Selective-breeding/selection criterion (High vs. Low vulnerability) | Behavior | Sweet substance/drug | Reference |
|---|---|---|---|
Novel environment reactivity (HR vs. LR)a | Operant responding (HR > LR) | Sucrose | (70) |
Impulsivity (5CSRTT; HiI vs. LoI)a | Operant responding (HiI > LoI) | Sucrose | (71) |
Active-avoidance learning (RHA vs. RLA)b Roman high vs. low avoidance | Consumption (RHA > LHA) | Saccharin | (72) |
Ethanol consumption (HAC vs. LAC)b | Consumption (HAC > LAC) | Saccharin | |
Consumption (HAC > LAC) | Sucrose | (75) | |
Ethanol consumption (HAC vs. LAC)a | Consumption (HAC > LAC) | Saccharin | (77) |
Sweet preference (SL vs. SDL)a | Consumption (SL > SDL) | Ethanol | (78) |
Self-administration | Amphetamine | (79) | |
Self-administration (SL > SDL) | Morphine | (80) |
Similar results have been found in clinical studies. Avidity for sweet consumption is positively related to drug abuse in humans. For instance, cocaine (81), nicotine (82, 83), opioid (84), and alcohol (85–87) users experience greater hedonic effects of sweetened dietary substances than nonaddicts.
Similar to addiction vulnerability, response to sweets also has a heritable influence (88–92). It has been proposed that these differences in sweet preference are not predominantly mediated by genetic variation in coding for peripheral taste processing (i.e., taste receptors), but for differences in reward processing that are related to central nervous system function (93, 94). Further, both alcohol-naïve individuals and alcoholics with familial histories of alcoholism display greater sweet preference than those without family histories of alcoholism (95). Taken together, these results display a clear relationship between sweet intake and addiction vulnerability that strongly implicates some shared genetically mediated biological mechanisms. Selectively breeding rats for differential saccharin intake has allowed us to more directly examine this relationship.
2.9.3 Selective Breeding for High or Low Saccharin Intake (HiS, LoS)
While investigating genetic influence on variability in response to sweets, Nachman (96) was the first to illustrate the heritability of saccharin preference by employing a selective breeding program in which rats were mated based on consumption of a saccharin solution. Subsequent experiments using inbred strains of mice further supported the heritability of saccharin preference (97–100); however, their avidity for drug intake was not investigated. Later, Dess and Minor (101) selectively bred rats (Holtzman Sprague-Dawley, Indianapolis, Indiana, USA) for high and low saccharin intake based on extreme saccharin phenotype scores that were derived from this measure:
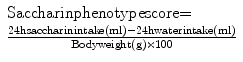
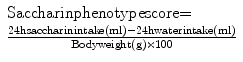
Originally, the resultant high and low saccharin-consuming lines, now called Occidental (Occidental College, Los Angeles, CA) HiS and LoS rats, were used to investigate the interaction between genetically mediated sweet preference and measures of emotionality. Subsequently, ethanol intake was compared in HiS and LoS rats, and as predicted, the HiS rats consumed more ethanol than LoS rats in free-choice and forced-consumption tests (67). These results prompted further investigation into differences in drug self-administration with the HiS and LoS lines. For example, Carroll et al. (20) showed that HiS rats and females acquired IV cocaine self-administration faster and in more animals per group than LoS rats or males.
A second colony of HiS and LoS rats was established from the Occidental HiS and LoS lines at the University of Minnesota, in which the primary interest was cocaine self-administration across various phases of the addiction model, although other drugs (e.g., heroin) and assays of drug vulnerability (e.g., delay discounting, drug-induced locomotor activity, etc.) have been employed. These experiments have largely shown the HiS and LoS rats to have consistent drug-prone and drug-resilient profiles, respectively, across several phases of drug abuse (see review by (5)). For example, as shown in Table 4, HiS rats exceed LoS rats in all phases of drug abuse, from acquisition to maintenance (20), escalation (23, 108, 111), and reinstatement (23, 107).
Table 4
Summary of results from studies on selectively bred HiS and LoS rats and drug-related behavior
Behavioral model | Drug/reinforcer | Phenotype effects | Sex | Reference |
|---|---|---|---|---|
Acquisition | Cocaine | HiS > LoS | M only | (20) |
Adoles vs. adult | Cocaine | HiS > LoS | M only | (102) |
Maintenance | Heroin | HiS > LoS | F > M | (20) |
Cocaine | HiS = LoS | M = F | ||
Escalation | Cocaine | HiS > LoS | F only | (23) |
Impulsivity (Delay discounting) | Food | HiS > LoS | F > M | (105) |
Cocaine | HiS = LoS | F = M | (105) | |
Impulsivity (Response disinhibition) | Food | HiS = LoS | F = M | (106) |
Cocaine | HiS > LoS | F > M | (106) | |
Extinction | Cocaine | HiS > LoS | (23) | |
Reinstatement | Cocaine | HiS > LoS | F only | (23) |
Adoles nic exposure/adult coc | Cocaine | HiS > LoS | F only | (107) |
Adoles vs. adult | Cocaine | HiS > LoS | F only | (108) |
Drug-induced locomotor activity | Cocaine | HiS > LoS | F > M (HiS) | (103) |
Drug-induced locomotor sensitization | Cocaine | HiS > LoS (F) | F > M | (103) |
Dysregulation of dose selection | Cocaine | HiS > LoS | F only | (104) |
Treatment | ||||
Baclofen on escalation | Cocaine | LoS > HiS | F only | (108) |
Progesterone on escalation | Cocaine | LoS > HiS | F only | (110) |
Histamine punishment | Cocaine | LoS > HiS | F only | (109) |
Neurobiological studies C-fos | Cocaine inj | LoS > HiS | M only | (36) |
Orexin cell labeling in hypothal | Coc/Sal inj | HiS > LoS | F only | (109) |
In addition to drug self-administration, the HiS and LoS rats also display differential behavioral profiles on other addiction-related measures, such as novelty reactivity (101), cocaine-induced behavioral sensitization (103), and impulsivity for food and/or cocaine during a motor impulsivity task (106), and a delay-discounting choice task (105). Given that these are common features in human and animal addiction vulnerability research, the HiS and LoS rats provide an exemplary animal model of genetically mediated addiction proneness and protection. A review of experiments conducted on HiS and LoS rats (Table 4) substantiated the drug proneness and resistance of the HiS and LoS rats, respectively, and also showed that these characterizations may interact with other vulnerability factors such as sex, age, and impulsivity (5).
2.9.4 Monitoring and Selecting for Impulsivity, a Trail that Is Closely Associated and Additive with Sweet Preference and Drug Abuse
As indicated in Fig. 1, another factor that is a strong determinant of drug addiction is impulsive behavior (68, 112–115).
Impulsive Choice-Delay Discounting
Impulsivity is an individual characteristic that has many parallels with differences in sweet preference (e.g., HiS, LoS); however, there are also unique determinants of vulnerability to drug abuse. The method that has often been used to select animals for HiI and LoI is delay discounting, which is a choice between a small, immediate reward vs. a large, delayed reward. There are other variations, such as immediate high probability of reward vs. delayed low probability of reward. In the delay-discounting procedure the sessions consist of 15, four-trial blocks and last for 2 h or until rats complete 60 trials, whichever is first. The first and second trials are forced on the immediate and delay lever, the third and fourth trials are choice trials. Different stimulus conditions are associated with each lever, and the delay and immediate sides are reversed daily or every other day. At the start of the experiment, the delay of the large reward is set at 6 S, and thereafter, responses made on the delay vs. immediate lever result in an increase or decrease, respectively, by 1 S of the delay on the delayed side. Thus, the animal adjusts its delay by responding on either side, and at the end of the session the delays on all of the 30 choice trials are averaged to yield a mean adjusted delay (MAD) for the session. The MAD is the main measure of impulsivity, and based on a distribution of over 180 rats, empirical evidence suggested a bimodal distribution of delays. Thus, high impulsivity was defined as less than or equal to 9 S and low impulsivity was defined as greater than or equal to 13 S. Typically a food reward is used to screen rats for high and low impulsivity (21), with the total daily food allotment being adjusted after the delay-discounting session, but cocaine infusions (small vs. large doses) have also been used as rewards (21).
Selecting rats for HiI and LoI choice on a delay-discounting task (21, 69, 112) or on premature responding on a 5-CSRTT (68, 116) has led to the same degree of predictability of addiction-prone (HiI) and -resistant (LoI) behavior as described for the bred HiS vs. LoS lines (e.g., (21)). For example, male and female HiI rats acquired cocaine self-administration faster and with more animals per group than their LoI male and female counterparts (33). During long access to cocaine, HiI rats showed significant escalation, while LoI did not change (22). However, during the reinstatement paradigm, different results occurred with HiI and LoI compared to HiS and LoS rats. The HiI and LoI groups did not differ during the 2-h maintenance phase; however, there were differences during extinction opposite to the HiS and LoS rats, with the LoI rats being more resistant to extinction than the HiI rats (33).
When HiS and LoS rats were tested on delay-discounting tasks for food or IV cocaine infusions (21), HiS males and females were more impulsive than LoS males and females, and females were more impulsive than males. Thus, vulnerability factors of saccharin preference (i.e., HiS), impulsivity (i.e., HiI), and sex (i.e., female) together form the most vulnerable condition. However, when cocaine was the reward in the delay-discounting task, there were no sex or saccharin phenotype differences. This is probably due to floor effects, since the MAD scores were in the HiI range in all groups.
In another procedure, the Go/No-go or signaled reward and nonreward task, rats were compared on alternating Go components, when a signal indicated food or drug reward, and No-go components when a different stimulus signaled nonreward. The measure of impulsivity was number of responses that occurred during the signaled nonreward component (No-go) (106). Using this task for food reward revealed no differences in impulsive action in HiS vs. LoS or males vs. females. Although it depends on the sensitivity of the impulsivity task, it appears that there is some overlap between saccharin preference and impulsivity.
The results obtained with HiS and LoS rats are similar to those found with another vulnerability factor, age. Adolescent rats showed higher Go/No-go responding than adults (110) when tested as adolescents and later as adults, and compared to adults that had been tested twice as a control. Adolescents, like HiS rats, also acquire cocaine self-administration faster, escalate their intake at a higher rate, show resistance to extinction, and reinstate more to a cocaine priming injection than adults or LoS rats. With regard to natural rewards, adolescent rats exhibited greater positive taste reactivity to (117) and consumed more of 6% (118) and 10% (117) sucrose solutions compared to adults. Thus, adolescence and high sweet preference are both prominent vulnerability factors, and may produce even more pronounced effects on drug seeking.
Ineffective Responding as a Possible Measure of Impulsivity in HiS vs. LoS Rats
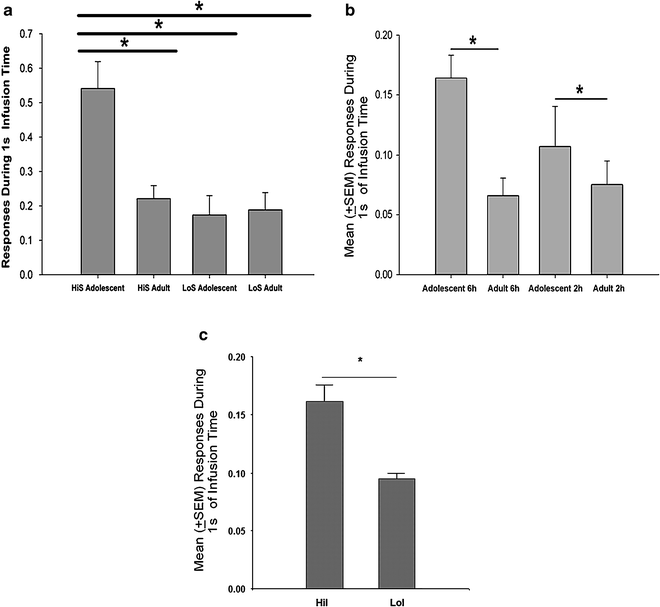
In our laboratory, rats typically self-administer IV drug solution under fixed-ratio schedules of reinforcement wherein a single active lever press results in a single infusion. Thus, active lever responses are followed by an infusion delivery interval during which responses are recorded but produce no additional infusions. These responses during the duration of the infusion are considered unreinforced responses (119) or ineffective responses (47). In recent studies of individual differences such as HiS vs. LoS, HiI vs. LoI, and adolescents vs. adults, an additional behavior that has been noted in the more vulnerable phenotypes is responding during the drug infusions. Ineffective responses were found by Kosten et al. (47) at low cocaine doses (0.0625, 0.125 mg/kg) in Fischer 344 rats but not at higher doses (0.25–1 mg/kg) or in Lewis rats. They suggested that since their Fischer rats maintained cocaine-reinforced behavior at higher levels than Lewis rats (although Lewis rats acquire faster), this might be a reflection of more craving in Fischer rats. Cummins and Leri (119) found ineffective responding with heroin self-administration but not at a 0.5 mg/kg dose of cocaine, and they interpreted the result as an elevation in motivation to obtain the drug. Ineffective responding has been found to be greater in HiS than LoS rats in our laboratory (with FR 1 schedules of 0.4 mg/kg cocaine reinforcement), in adolescents than adults, and in HiI than LoI rats (see Fig. 2). Since ineffective responding occurs in groups that self-administer more cocaine than their lower vulnerability counterparts, and elevated responding is not consistently found on the inactive lever in the highly-vulnerable rats, it is assumed that this behavior is directly related to the reinforcing effects of the drugs and stimulus properties of the manipulanda (lever) associated with drug reward. In this sense, it may be a form of sign tracking that is associated with elevated drug self-administration in rats selected for behavior directed toward the CS (sign-lever) (121). Other potential explanations suggested increased motivation because ineffective responding increases at lower drug doses (that may have a priming effect), under PR schedules (47, 119) that assess motivation, and during food restriction that increases motivation (119, 122, 123). Ineffective responding may also be an indicator of elevated impulsivity, as it is higher in rats selected for high (vs. low) impulsivity (Fig. 2d), and rats selected on other vulnerable features, such as HiS (21) or adolescents (111) also show elevated impulsivity.
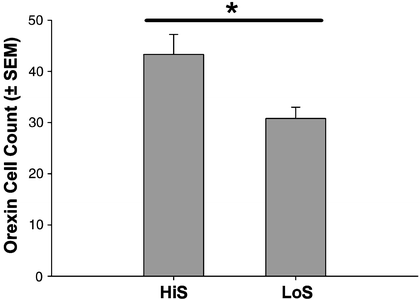
Additional evidence that the HiS rats may have higher motivational status than LoS rats is from a neurobiological analysis of HiS and LoS rats that examined the number of orexin-positive cells in the lateral hypothalamus. Figure 3 indicates that these cell counts were higher in the HiS than the LoS rats (e.g., ineffective responding), supporting the behavioral data that suggest higher motivation levels. Orexin-1 is a neuropeptide that stimulates the motivation to ingest preferred substances and mediates dopamine release that affects motivation for cocaine and other highly valued rewards. Interestingly, orexin administration increased saccharin intake in rats, and mRNA expression of orexin increased following saccharin consumption (124). Further, the orexin-1 antagonist blocks motivation for highly preferred rewards, such as high-fat chocolate in rats (125) and craving and reinstatement (126). These data suggest that differences in the distribution of orexinergic neurons between the lines may contribute to a variety of differences in reward-seeking behavior, including the phenomenon of ineffective responding.
3 Notes
3.1 Assessing Responsiveness to Aversive Effects of Drugs in HiS vs. LoS Rats
Previous work has shown that LoS rats display greater negative reactivity, or emotionality, compared to HiS rats under stressful conditions (101, 127). For example, LoS rats show greater latency of emergence and increased defection in the novel open field, and more stress-induced anorexia (101) and analgesia than HiS rats (127). Further, compared to HiS rats, the LoS animals display increased reactivity of the hypothalamic–pituitary–adrenal (HPA) axis, a system that is integral to the stress response and linked to a multitude of psychiatric disorders, including drug addiction (128, 129).
As stress is a powerful liability in many aspects of substance dependence (129), the HiS and LoS model may have special utility in investigating a more broad construct of emotionality that can, along with results from other high- and low-vulnerable animal models (see Table 5), provide an overarching framework with predictive and translational value. The following studies are examples of the interactions between genetic differences in drug abuse vulnerability (HiS vs. LoS) and several measures of stress reactivity.
Table 5
Relationship between high and low responders for drug and nondrug rewards and response to aversive conditions (adapted from (17))
Groups | Positive effects | Negative effects | Reference |
|---|---|---|---|
HiI vs. LoI (impulsivity) | HiI > LoI | LoI > HiI Cocaine extinction | (33) |
LoI = HiI | (36) | ||
Heroin withdrawal | (116) | ||
Cocaine withdrawal | (116) | ||
HiS vs. LoS (saccharin intake) | HiS > LoS | LoS > HiS | |
Ethanol withdrawal | |||
Glucose withdrawal | |||
LoS > HiS | |||
Food deprivation-induced wheel running | |||
LoS > HiS Acoustic startle | (127) | ||
LoS > HiS | (131) | ||
Food-deprivation + methylphenidate-induced startle | |||
LoS > HiS | (109) | ||
Punishment of cocaine intake | |||
HR vs. LR (novelty reactivity) | HR > LR | LR > HR Fear, anxiety and emotionality | |
HAC vs. LAC (ethanol intake) | HAC > LAC | LAC > HAC Ethanol withdrawal | (134) |
LEW vs. F344(inbred strains) | LEW > F344 | F344 > LEW | (135) |
Fear, anxiety, emotionality, F344 > LEW | |||
Taste aversion (morphine) | (18) |
3.2 Food Restriction-Induced Hyperactivity
One stressful condition, acute food restriction, increases wheel running more in LoS rats compared to HiS rats ((127); Zlebnik and Carroll, unpublished observations). In the rat’s natural environment, this increased activity is considered paradoxical because the animal is expending extra energy when caloric resources are scarce. However, hyperactivity also promotes foraging behavior and increases the probability of discovering new sources of food. Recently, McLaughlin et al. (131) investigated the interaction of restriction-induced hyperactivity in the running wheel and the effects of methylphenidate, a stimulant that activates the mesolimbic dopamine system and increases HPA activation (136) and increases wheel running (137). The LoS food-restricted group treated with methylphenidate showed the most wheel running compared to HiS and vehicle-treated groups. These results indicated that methylphenidate enhanced the relatively elevated restriction-induced locomotor activity in the LoS rats, but not in the HiS rats. Similarly, wheel running was elevated in chronically food-restricted LoS rats compared to HiS and food-satiated groups ((127), Zlebnik and Carroll, unpublished data; Fig. 4). This enhancement of the already elevated emotional reactivity in the LoS rats may be attributable to the augmentation of HPA functioning.
3.3 Food Restriction- and Withdrawal-Induced Acoustic Startle
The startle response is a defensive reflex that follows an acute, salient stimulus (e.g., brief, loud noise; acoustic startle), and its amplitude reflects internal affective states, such as anxiety (138). Startle can be modulated by environmental and intrinsic variables or exogenously administered (pharmacological) agents (139–141). Recent studies have used this paradigm to associate the differential reactivity to aversive conditions in the HiS and LoS rats to their respective differences in drug abuse vulnerability. An initial study by Dess et al. (127) showed that LoS rats exhibited greater startle amplitude in response to brief, intermittent bursts of white noise (acoustic startle). A recent study expanded these findings by investigating the effects of stress in the form of inescapable foot shock on startle amplitude between the phenotypes (142). Greater aversive effects in LoS (vs. HiS) rats were also indicated in another recent experiment from Mclaughlin et al. (131) that found that food-deprived LoS rats treated with methylphenidate had greater acoustic startle compared to those treated with saline, while these results were not found with the HiS rats. These studies illustrate that rats with distinctive drug vulnerability profiles also show divergent responses to stressful events, suggesting that emotional reactivity may modulate the aversive aspects of drug administration (toxicity, withdrawal) and strongly impact addiction liability.
Another approach to assessing the aversive aspects of commonly abused drugs is to compare withdrawal effects between HiS and LoS lines. Withdrawal is a negative, anxiety-like affective state that involves, in part, the activation of the HPA axis (143). Dess et al. (130) investigated the effects of withdrawal 24 h following 2 weeks of chronic, forced ethanol exposure on an acoustic startle response in the HiS and LoS rats. The LoS rats exposed to ethanol had greater startle amplitude than LoS rats with access only to water and HiS rats exposed to ethanol or water.
Withdrawal-like responses in the HiS and LoS rats were recently extended to the effects of forced glucose abstinence (66). Because drug dependence, dysregulated food intake, and food addiction show strong parallels (144–146), strain differences could provide deeper insight into variance in emotionality by examining nondrug rewards. In this experiment, rats were given extended access to a glucose solution and then glucose deprived for 1 day until the acoustic startle response was measured. While there were no line differences in startle between rats that received glucose, escalation of glucose intake was correlated with increased startle in the LoS group. Combined, these results are in line with the previous experiments showing that HiS rats displayed elevated intake (binging) of ethanol and other drugs, while LoS rats showed more severe withdrawal effects and were more sensitive to the aversive aspects of chronic drug exposure, providing a partial explanation of their relative reduction in drug-seeking behavior.
Recent preliminary work with punished drug self-administration has provided additional evidence to this end. In this study, HiS and LoS rats maintained a period of stable IV cocaine self-administration, then histamine was added directly into the cocaine solution. Systemic histamine administration is an effective punisher that reduces cocaine self-administration in monkeys (147, 148). Therefore, to investigate strain differences in drug-seeking behavior in spite of aversive consequences, a defining behavioral characteristic of addiction, a primary function of histamine in this experiment was to act as a contiguous, interoceptive punishing agent. Following this assessment, the cocaine/histamine solution was replaced with a histamine-free cocaine solution, and a third self-administration phase commenced. Results indicate that, compared to baseline, histamine reduced self-administered cocaine infusions equally in both strains. However, when histamine was terminated, HiS rats returned to higher levels of cocaine self-administration at a faster rate, while LoS rats remained significantly suppressed for up to 20 days (Fig. 5a). These data suggest that the LoS rats are more sensitive to histamine’s punishing effects than HiS rats.
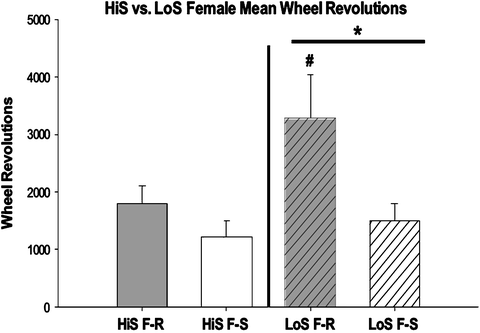
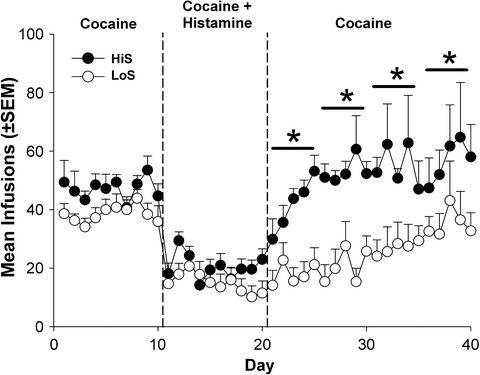

Fig. 4.
Wheel revolutions in HiS and LoS rats that were food restricted (F-R) or food satiated (F-S). *p < 0.05, LoS F-R vs. LoS F-S and #p < 0.05, Los F-R vs. HiS F-R. From Zlebnik and Carroll, unpublished observation.

Fig. 5.
Punishing effects of adding histamine to i.v. cocaine self-administration solution in HiS and LoS rats. *p< 0.05 HiS vs. LoS. From Holtz et al., unpublished observation.
3.4 Examining Individual Differences in Responsiveness to Treatment
Recent evidence suggests that the individual differences that have been reported for many aspects of drug abuse also extend to differences in responsiveness to treatment. Most treatment studies regarding individual differences involve males and females, as summarized in Table 6. For example, spiradoline, a κ-opioid agonist, produced a greater reduction in cocaine-induced locomotor activity in female mice compared to males (149), and a similar drug, bremazocine, decreased phencyclidine self-administration under a FR schedule to a greater extent in female rhesus monkeys compared to males (152). Furthermore, the gamma-aminobutyric acid-B (GABAB) agonist, baclofen, had a greater effect of lowering acquisition rates of IV cocaine self-administration in female rats compared to male rats (150), and ketoconazole, a corticosterone synthesis inhibitor, suppressed heroin self-administration more in female than in male rats (155). Sex differences in responsiveness to treatment for drug abuse also extend to non-pharmacological interventions. For example, access to a running wheel significantly decreased cocaine self-administration in female rats but not in males (151), and access to saccharin reduced phencyclidine intake to a greater extent in female compared to male monkeys (153).
Table 6
Individual differences in sex and age and treatment effects
Behavioral model | Drug | Treatment | Treatment effect | Reference |
|---|---|---|---|---|
Locomotor activity | Cocaine | Spiradoline | F > M | (149) |
Acquisition | Cocaine | Baclofen | F > M | (150) |
Self-administration | Heroin | Ketoconazole | F > M | (16) |
Cocaine | Exercise | F > M | (151) | |
Phencyclidine | Bremazocine | F > M | (152) | |
Phencyclidine | Saccharin | F > M | (153) | |
Cocaine | Histamine | F = M | (109) | |
Escalation | Cocaine | Exercise | Adoles > adults | (154) |
Reinstatement | Cocaine | Cocaine hydrolase | F = M | (110) |
Further studies have shown consistent findings that many aspects of drug abuse are reduced in female rats, monkeys, and humans by treatment with progesterone or its metabolite allopregnanolone, and there are no effects in males, as these are ovarian steroid hormones (see reviews in (16, 156–158)). While the scope of individual differences in these examples is limited to sex, together they offer an experimentally tractable link between neurobiological differences that underpin both addiction severity and treatment sensitivity.
3.5 Treatment of Drug Abuse Modeled in HiS and LoS Rats
While the HiS and LoS rats also represent groups that consistently differ in responsiveness to the positive and negative effects of drugs and vulnerability to addiction, little is known about how these treatments affect rats with these individual differences. In a previous study, Garbutt et al. (159) showed that severe alcoholics with a preference for high concentrations of sucrose responded more favorably to naltrexone treatment of alcoholism than those preferring lower concentrations. Recently, selectively bred HiS and LoS and selected HiI and LoI rats have been tested with treatments that have been shown to reduce drug intake without affecting food-maintained behavior (e.g., baclofen, progesterone) or treatments that involve punishment of drug-maintained responding (e.g., histamine).
In the initial studies, a long access (6 h) escalation procedure was used to examine the effects of treatments in HiS and LoS rats. This procedure is considered an animal model of drug bingeing in humans, and it is sensitive to individual differences, including sex (24, 160), and saccharin intake, such as that displayed by the HiS vs. LoS rats (23), and impulsivity in selected HiI and LoI rats (22). In these studies, females exceed males, HiS rats exceed LoS rats, and HiI rats exceed LoI in escalation of their cocaine intake over a 21-day period. Escalation is considered to be a critical component in the transition from controlled drug use to uncontrolled use and addiction; it is mediated by dramatic shifts in mesolimbic reward system functioning (143), and it is considered to be target for treatment approaches. However, only a few studies have addressed the use of treatment agents during this phase (e.g., (161, 162)), and none have evaluated individual differences using selective breeding models.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree