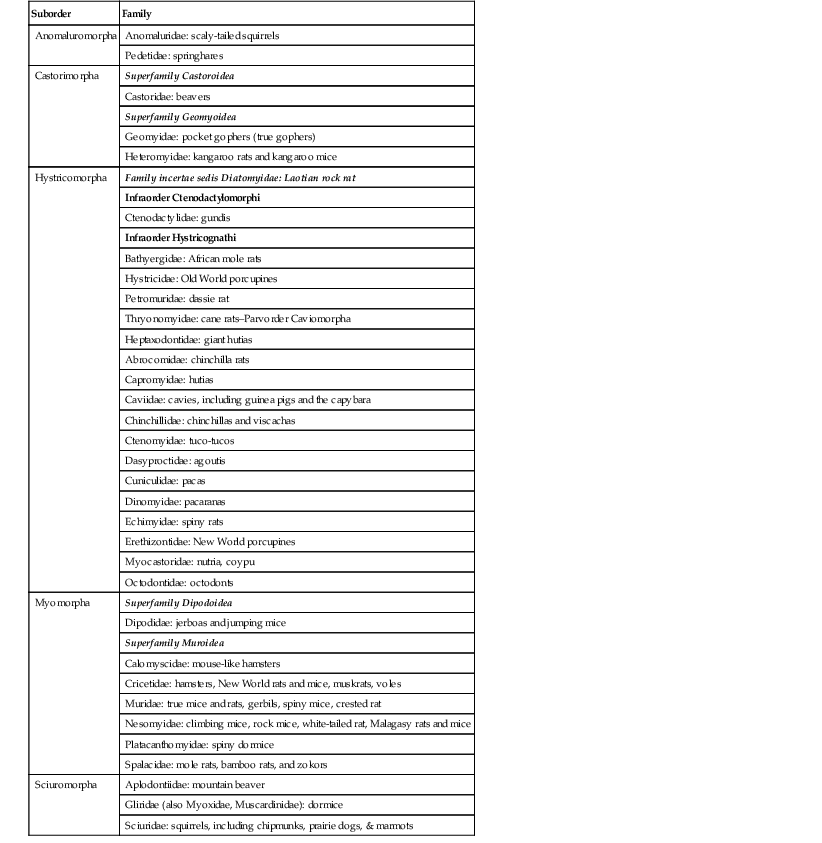
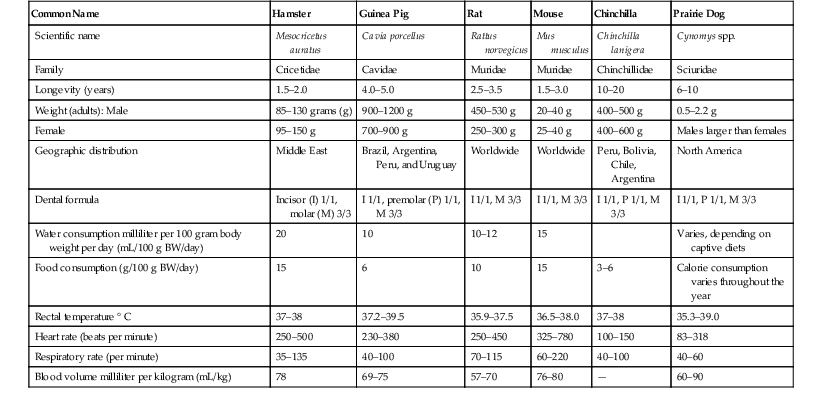
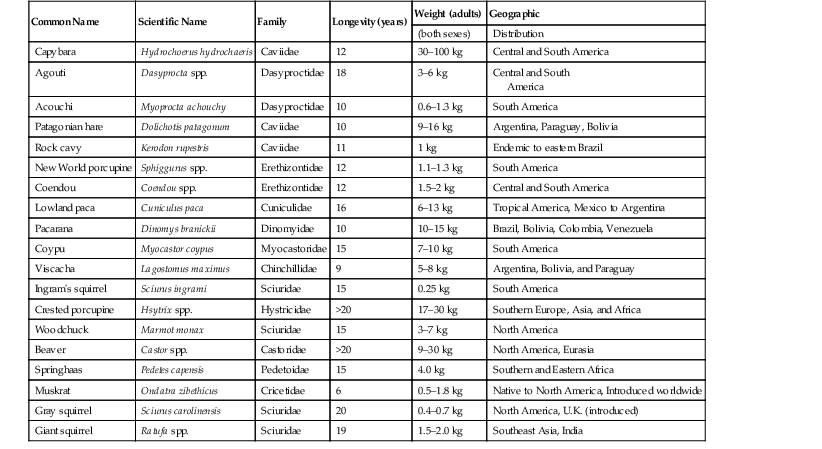
Enrique Yarto-Jaramillo Rodentia, being represented by nearly half of all placental mammals, is therefore the most diverse animal order comprising 2277 species divided into 33 families. Phylogeny at the family level is still being debated, and more molecular and morphologic studies are needed.52 Rodent classification is based on morphologic features according to two different systems. Brandt has divided rodents on the basis of the position of masticatory muscles (masseters) into three suborders: Myomorpha, Sciuromorpha, and Hystricomorpha,26 whereas Tullberg has divided rodents into two suborders known as Sciurognathi and Hystricognathi, according to the position of incisors and the angle of the jaw.60 Box 42-1 describes the current classification of the order Rodentia. Nonetheless, molecular analyses have placed rodents into seven clades: (1) Anomaluromorpha (scaly-tailed flying squirrels, springhares); (2) Ctenohystrica (gundi, porcupines, guinea pigs); (3) Castoridae (beavers); (4) Geomyoidea (pocket gophers, pocket mice); (5) Myodonta (rats, mice, jerbos); (6) Gliridae (dormice); and (7) Sciuroidea (mountain beavers, squirrels, woodchucks). More recent studies have performed phylogenetic reconstructions that allow the division of those seven clades into three lineages: (1) a “squirrel-related” clade (Sciuriodea and Gliridae); (2) a “mouse-related” clade (Anomaluromorpha, Castoridae, Geomyoidea and Myodonta); and (3) Ctenohystrica.52 Rodents are naturally found in all continents except Antarctica, inhabiting diverse environments with different living adaptations such as semi-aquatic, terrestrial, arboreal, fossorial, jumping, and gliding.5 Rodents may be found from the high arctic tundra to equatorial rain forests, temperate bogs and swamps to hot arid deserts, mountain tops to sandy canyon bottoms.64 In South America, rodents are the most numerous in species and in abundance compared with other continents, corresponding to approximately 44% of the total amount of native mammals.48 In Australia, all of the more than 60 species of rodents belong to the family Muridae, which is the most widespread of all mammalian families in this continent. In recent literature, five groups of rodents (Pseudomys, Uromys, Hydromys, Xeromys, and Pogomomys) are frequently called the “Old Endemics,” referring to the groups that entered Australia at the very end of the Miocene period, approximately five million years ago. True rats are often referred to as the “New Endemics,” since they arrived in Australia around one million years ago.6 Sixteen of the extant Australian rodents are currently threatened (International Union for the Conservation of Nature [IUCN], 2006), all belonging to the Old Endemics.6 In general terms, rodents are characterized by small and compact bodies that are cylindrical to spherical in shape, and short legs; they weigh from less than 10 grams (g) to more than 66 kilograms (kg).6,64 Hamsters and other rodents have extensive distensible cheek pouches (evaginations of the oral mucosa) that are used to carry food, bedding material, and occasionally the young. Gerbils possess strong claws for burrowing and muscular legs for jumping and standing.51 Chinchillas have soft fur with 60 hairs per follicle; on either side of the upper lip, they have long vibrissae that are used as sensory organs. Duprasi (Pachyuromys duprasi) have developed claws on the front feet and a fleshy tail. Degus are similar anatomically to both guinea pigs and chinchillas. Guinea pigs have large tympanic bullae and four digits on the forelimbs and three digits on the hindlimbs.27 Hamsters, rats, and mice possess four front toes and five hind toes. Porcupines have a distinct integument feature as modified hairs that are stiff spines known as barbed quills among guard hairs on the back and tail. Porcupines also have thin skin that will tear with the slightest pressure.42 Some rodents such as hamsters have flank glands in the form of dark brown patches, which are used to mark their territory and as sexual stimulation. Several tail adaptations are present in rodents for a wide variety of functions: fat storage in rock rats (Zyzomis spp.), a long tail providing balance when hopping in some mice (Notomys spp.), and the ability to shed tail skin when seized by a predator in hopping mice, or a prehensile tail to aid in climbing in the prehensile-tailed rat (Pogonomys spp.).6 All rodents possess two pairs of continuously growing incisors that confer the ability to gnaw, as well as a monophyodont dentition (no primary teeth are present).32 They also have a diastema space with no teeth between incisors and molars on each dental arcade. Rodents are divided in two groups on the basis of dentition. Dentition in Muridae (mice, rats, hamsters, and gerbils), Sciuridae, Castoridae, Erethizontidae, and Myocastoridae is classified as elondont (grow continually) only for the incisor and as brachydont (closed-rooted) for the molars.51 The brachydont group of rodents consumes diets that are high in caloric energy and not sufficiently abrasive, so their teeth are low crowned with well-rooted premolars and molars. The second group, hystricomorph rodents (“porcupine-like”), including guinea pigs, chinchillas, degus, New World porcupines, agoutis, and others have all teeth open-rooted which grow continually.27 This group comprises herbivorous rodents whose teeth have evolved to grind abrasive more voluminous diets, as their teeth have large chewing surfaces.32 The dental formulas of rodents vary according to different taxonomic families, although in general terms they possess four incisors (I), no canines (C), few premolars (P), and 8 to 12 molars (M). Old world rats and mice (Muridae) have 16 teeth (2 I 1/1, C 0/0, P 0/0, M 3/3), whereas squirrels (Sciuridae) have 20 to 22 teeth (2 I 1/1, C 0/0, P 1-2/1, M 3/3). Other rodent families (Caviidae [guinea pigs]; Chinchillidae [chinchillas]; Hydrochoeridae [capybaras] and Castoridae [beavers]) possess 20 teeth (2 I 1/1, C 0/0, P 1/1, M 3/3).32 Rodents do not possess canine teeth, and the Australian species also lack premolars, so the dental formula for these later rodent species is: I 1/1, C 0/0, P 0/0, M 2-3/2-3.6 Voles are an intermediate group with variation in crown length between species. Incisors in rodents differ from other teeth. Enamel is primarily present on the labial surface and is deposited unevenly. The enamel of most common rodents is white, but some species (chinchillas, hamsters, and others) may have enamel that is orange to yellow in color. Rodents have a simple stomach with glandular portions, whereas hamsters and other rodent species possess a nonglandular portion (pars cardiaca) or forestomach and a glandular stomach (pars pilorica). Rats have no gallbladders. In agoutis (Agouti paca), guinea pigs (Cavia porcellus), coypus (Myocastor coypus), and capybaras (Hydrochoerus hydrochahaeris) lung anatomy is well described, which might be useful for clinicians to diagnose the location of pulmonary disease and when performing necropsies.13 The urinary and reproductive tracts terminate in separate urethral and vaginal orifices in the female. Small rodents are spontaneous ovulators and are polyestrous. Chinchillas have two cervixes and two uterine horns. In most female rodents of both suborders, the genital opening is opened only during the heat season, after parturition, and when an infection occurs.48 Most male rodents have open inguinal canals, an os penis, and a complex urogenital system that contains prominent accessory glands.33 Males of the Dasyproctidae family possess a pair of keratinized lateral accessory structures on the glans in the form of “wings.”48 The testes usually lie in the scrotum in the breeding season; however, in porcupines, agoutis, chinchillas, cavies, and capybaras, testes lie in the inguinal canal, so no true scrotum is present in these species. A distinct scrotal sac is absent in prairie dogs. One of the most important features to consider in rodent breeding is the precocial nature of the youngsters in the suborder Stricognatha, whereas they are altricial in the suborder Scuirognatha.48 Prairie dogs possess a unique anatomic feature: trigonal anal sacs, which are ducts that appear as white papillae beside the anus.28 Female house mice have five or more pairs of teats, whereas female Australian native mice have only two pairs of teats, located in the inguinal region.6 Tables 42-1 through 42-3 provide biologic information on New World and Old World rodents. TABLE 42-3 Biologic Data of the Most Common Australian Rodents Kept in Captivity* Rodents do not pant and have no sweat glands, so their ability to withstand high temperature is very limited. Hamsters, chipmunks, and prairie dogs are permissive hibernators. Other rodents such as woodchucks (Marmota monax) do hibernate, presenting an annual cycle of changes in metabolic function during the winter. When ambient temperatures reach 5° C (41° F), they curl up and enter a deep sleep.33 Prairie dogs may enter a dormant period in inclement weather and tend to gain weight in the autumn as the light cycle and temperature decrease.28 Rodents are monogastric, and most practice some degree of coprophagy or cecotrophy, a behavior that involves ingestion of pellets of digestive origin taken directly from the anus, for two reasons: (1) to repopulate the intestinal tract with bacterial flora and (2) to absorb amino acids and vitamins B and K synthetized by those microbes. Cavies, chinchillas, porcupines, voles, beavers, capybaras, lemmings, and muskrats and other rodents all have complete glandular stomachs, are strict herbivores, and are cecotrophic. They have a prominent cecum and an elongated colon and are hindgut fermenters; bacterial and protozoal fermentation aids in the digestion of cellulose.6,50 Carnivorous species such as the water rat (Hydromys chrysogaster) have a relatively small stomach, with most (74%) of this organ being composed of glandular tissue.6 In rats and hamsters, a muscular sphincter limiting the esophagus and stomach prevents these species from vomiting. Insectivorous rodents (grasshopper mice, Onychomys sp.; burrowing mice, Oxymycterus sp.) have unpocketing and special glandular areas in the stomach.50 Rodents in the family Muridae and Cricetidae are omnivorous and coprophagic and have a simple stomach, small intestine, and modest development of the colon and cecum, all of which permits some degree of retention of ingesta for fiber fermentation. Smell is probably the most important sense in most rodents, since the reproductive cycle, sexual attraction, and parental care are influenced by the acrid odors from glandular secretions. In dasyproctids, a pair of glandular structures in the perianal area secrete acrid odors to mark territory and as a means of sexual communication.48 Being such a diverse animal group, rodents have different housing requirements, and most are nocturnal. Among nocturnal rodents, we find the following families: Pedetoidae, Hystricidae, Castoridae, Agoutidae, Chinchillidae, and Dinomyidae.50 Australian rodents may be arboreal, terrestrial, aquatic, and burrowing. Rodents are agile and fast, jump high, burrow, and gnaw, and it is of utmost importance to consider these adaptations when designing an enclosure for this animal group, particularly with regard to cleaning.6 Rodents have been housed singly, in pairs, or in harem groups, although some of them do well in groups, as they are social animals living in large communities in the wild (prairie dogs and capybaras).28,48 Diurnal species of rodents include Sciuridae, Caviidae, Myocastoridae, Dasyproctidae, and Octodontidae. Capybaras are crepuscular rodents that live in groups of more than 20 animals, with a dominant male and several females.48 Agoutis and acouchis (Myoprocta achouchy) are examples of South American terrestrial rodents, and coypus (Myocastor coypus) capybaras and beavers are aquatic species.48 Australian rodents are mainly nocturnal or crepuscular, so for display purposes, reversed light cycles are used. It is worthy to mention, however, that some rodent species in captivity show a partial shift from nocturnal activity to diurnal activity.6 Species of terrestrial (prairie dogs) or arboreal (common dormouse, Muscardinus avellanarius) rodents that hibernate or estivate (Sciuridae, Castoridae, Hystricidae, and Chinchillidae) require burrows, nest boxes, and even hollow logs or other types of materials.50 Shelter and nest boxes should include rock shelters, tussock grasses, and plastic boxes.6 For some smaller species of rodents (hamsters, degus, duprasi, chipmunks, prairie dogs), it is advisable to provide deep bedding made of materials such as newspaper, shredded recycled paper pellets, or hardwood shavings for digging and tunneling. Sawdust or sand makes a good substrate for energetic burrowers. Coarse sand may be abrasive, so fine-grained sand is recommended.6 The printing ink in newspapers is toxic to hamsters.28,51 The design of enclosures for rodents depends on the species. Enclosure size must reflect the natural behaviors of the species (arboreal or terrestrial).6 For domesticated rodents, a minimum space of 0.9 square meters (m2) per adult (guinea pigs, chinchillas) is recommended. Cages must be made of strong metal, not wood, and should not be larger than 2.5 centimeters (cm), and have fine wire meshing to prevent foot and limb injury. The cage should have a metal support structure, with part of the floor being solid. For smaller terrestrial species, a floor space of 50 × 40 cm is recommended, and for larger terrestrial species such as Australian greater stick-nest rat (Leporillus conditor), the minimum recommendation is 200 × 200 cm.6,28 The cage must be spacious enough for exercise and grazing, according to each species’ needs, requiring both horizontal and vertical space with multi-levels and shelves (for chinchillas, New World porcupines, and many zoo rodents).27,28 Vertical space of up to 2 m is recommended for some arboreal species, and Australian water rats (Hydromys chrysogaster) require a minimum space of 300 × 300 cm and a pond of 100 × 100 × 50 cm depth.6 Many species of zoo rodents burrow, so to prevent escapes from outdoor facilities, it is recommended that the fence be strong, made of concrete or chain-link, and have foundation at least 1 m below ground.50 Some rodent species (chinchillas, guinea pigs) are prone to heat stroke at temperatures higher than 25° C and high humidity (>60%). The preferred housing parameters for these species are environmental temperature between 10° C and 24° C and 30% to 60% humidity.27 Enclosure location must be based on environmental temperatures and ventilation, so enclosures should not be placed in direct sunlight coming in through a window to prevent overheating.6 To avoid buildup of ammonia, enclosures must be well ventilated and easy to clean. Frequency of cage cleaning varies according to the density of animals, substrate type, and levels of stress that the animals inside the enclosure experience with this activity. Porcupines are very sensitive to high environmental temperatures. Shade should be provided in extreme weathers (hot and humid days). Cold and damp conditions are also detrimental for this and other tropical rodent species. Maintaining appropriate conditions in the enclosure is advised. For most small rodent species a normal light cycle (14-hour light, 10-hour dark) is advised.59 It is important to note that Dasyprocta species prefer eating on elevated surfaces or under and within vegetation, so food items must be placed accordingly for captive agoutis.40 Furniture for rodent cages or enclosures also depends on the species housed indoors or outdoors and includes exercise wheels, apple or maple branches, PVC pipes and fittings, materials for gnawing, ponds and pools (muskrats, capybaras, beavers, coypus). In captivity, beavers (Castoridae) need a pool with tree branches to build a lodge. Coendus, squirrels, chinchillas, and New World porcupines are arboreal and require enclosure furniture that allows them to climb. Some rodents (Patagonian cavies, agoutis, acouchis) may jump up to 2 m, so enclosure walls must be tall enough to prevent escape. Double doors with secure locking mechanisms are recommended for zoo rodents.48,50 Species of Australian rodents from arid habitats tend to nest communally, whereas most species of southern Australia and tropical forest live in smaller groups or are solitary, and this must be considered when designing enclosures for these types of rodents.6 Diets for free-living rodents are as diverse as this animal order. Many rodents consume digestible foods such as nuts and seeds, but other rodents may depart from the omnivorous pattern.31 The rodent gastrointestinal (GI) tract shows great diversity, indicating specialization for a variety of diets. Rodents have herbivorous and carnivorous feeding adaptations, although the majority of rodents are omnivorous. Capybaras, rock cavies, and chinchillas in the wild feed preferentially on plants, grasses, and barks of small shrubs and bushes. Their natural diet is high in fiber and low in energy content. Capybaras, coypus, and beavers feed preferentially on aquatic plants, which are eaten in the water.48 Agoutis and acouchis in the wild eat shoots, flowers, fruits, fungi, and roots, whereas coendus, New World porcupines, and squirrels, which are arboreal, feed on leaves, flowers, shoots, and palm tree fruits at tree canopies.48 However, evidence supports the classification of the Dasyprocta species as a frugivore. Dasyprocta leporine is reported to have a wild diet of 87% fruit, 6% animal matter, 4% fibrous foods, and 2% leaves. Currently, any evidence of cecotrophy in agoutis is lacking.40 Wild prairie dogs eat grasses, prairie herbs, and weeds. Under natural conditions, prairie dogs have shown three deviations from herbivory: (1) They consume fresh and old scats of American bison; (2) these rodents sometimes also eat insects such as cutworms (Noctuidae) and ground beetles (Carabidae), and short-horned grass-hoppers (Acrididae); and, (3) prairie dogs sometimes cannibalize other prairie dogs that have died of natural causes or other unweaned prairie dogs.25 In the wild, Patagonian cavies consume vegetation and fruits. They consume leaves of monocot species (70%) and dicot species (30%). Monocot species include Chloris, Pappophorum, and Trichloris, and the perennial dicots include Atriplex lampa, Lycium, and Propsis.30 Desert-living rodents eat succulent plants in the wild to obtain their water supply. Duprasi are omnivorous and eat a considerable amount of insects. Degus in the wild consume herbaceous foliage (60% by volume), with young plants and new leaves preferred over mature plants. Grasses and some seeds seasonally make up the remainder of their diet.27 The diet of tree squirrels (two genera, Sciurus spp. and Tamiasciurus spp.) is predominantly seeds (including nuts), fruits, buds, leaves, bark, and fungi. These rodents sometimes eat invertebrates such as beetles, caterpillars, and the larvae of various insects.20 Flying squirrels preferentially consume hypogeous mycorrhizal fungi (truffles) over other food items. They also feed on mast-crop nuts, tree sap, insects, carrion, bird eggs and nestlings, buds, and flowers.20 Australian water rats are carnivorous, and their natural diet consists of fish, yabbies, prawns, and mollusks, whereas central rock rats are granivorous, and bush rats (Rattus fuscipes) eat a great variety of food items such as fungi, leaves, insects, and seeds in nature.48 To meet the nutritional and behavioral requirements of a particular rodent species in captivity, it is best to provide a varied diet that reflects the natural diet.48 For domesticated rodents, many different commercial diets are available, although pelleted complete feeds are preferred over products that contain a mix of seeds and grains. Fiber digestibility in Patagonian cavies is apparently similar to that of guinea pigs, although the digestibility of crude protein and ash is reported to be lower in Patagonian cavies. Even though Patagonian cavies are closely related to coprophagous guinea pigs, it is not clear whether the cavies are coprophagous as well.30 Adapted diets for herbivorous zoo rodents contain a mixture of rodent pellets; high-fiber herbivore pellets (acid detergent fiber higher than 30%), which are low in starches and soluble carbohydrates, low in protein, and high in indigestible fiber; and grasses, vegetables, hays, and small amounts of fruits (Eduardo Valdés, personal communication). Bermuda-grass, brome, fescue, and timothy hay are examples of nutritious foods that may be offered to captive rodents.25 Clover and alfalfa hays are not recommended for some rodents (guinea pigs) because of their high calcium content, which may lead to calcification within the renal system in adult rodents.15 Diets of Patagonian cavies in zoo settings have been reported to consist of commercially produced rodent or primate chow diets, with the addition of leafy greens as the largest proportion, and some fruits.30 (It should be noted that primate chows, when used, should consist of higher-fiber and low-soluble carbohydrates.) Excessive levels of some fruits and vegetables (e.g., citrus fruits) may lead to GI disturbances in some rodent species, and excessive levels of high-energy foods (sunflower seeds, dog and cat food) may lead to obesity and nutritional imbalances.48 The high proportion of seeds and nuts in the diets of wild Dasyprocta indicate the need for the selection of foods higher in energy and lower in water content for captive animals to ensure sufficient protein and fats in the diet.40 Dietary fiber levels for wild Dasyprocta are apparently consistent year-round. In captive agoutis, the suggested level of dietary crude fiber for a 2.7-kg animal is 157 g crude fiber per kilogram dry matter (DM) feed.40 It must be remembered that high-fiber hays and grasses are crucial for captive rodents to ensure normal GI tract function, teeth wearing, and overall health, so they must be provided ad libitum on a daily basis.15,59 Rodents should be provided with suitable objects to gnaw to allow normal behavior and reduce the incidence of dental disease.48 Vitamin and mineral supplements are not required if a balanced (varied) diet is supplied to captive rodents. Unlimited access to clean, fresh water should be ensured for all rodents in captivity, even for desert-living species. Guinea pigs and other members of the family Caviidae (capybaras) are incapable of endogenous synthesis of vitamin C (ascorbic acid), since they have a mutated gene for l-gulono-g-lactone oxidase. This enzyme prevents the conversion of l-gulonolactone to l-ascorbic acid.22,65 Thus, the provision of supplemental vitamin C is an important component of the dietary management of guinea pigs in captivity. Classification of Dasyprocta species as omnivores supports endogenous ascorbic acid synthesis. A study on the biosynthesis of ascorbic acid in the acouchi and agouti further supports endogenous ascorbic acid synthesis in Dasyprocta.40 Interesting and useful information, including respiratory rate and effort, overall behavior, and gait, may be obtained by examining the rodent as it moves around on the examination table.33 Some rodent species may also become stressed and collapse during prolonged handling, so minimal handling is advised.6 All rodents, including some kept as pets, tend to bite, and improper handling may result in injury to the animal, the handler, or both. Also, handling of small rodents may be challenging, and the key is to maximize diagnostic information while maintaining the safety of both the clinician and the patient.23,33 Pet rodents may often be picked up in the palm of the hand or grasped by the tail for transferring or examination.23 Unlike domesticated rodents, grasping the tail of many Australian rodents may result in the tail skin slipping off or degloving of the tail.6 Small rodents such as mice, rats, hamsters, gerbils, duprasi, and chipmunks may be held around the shoulders and pelvic girdle, with the head between the first and second fingers. Firm but gentle pressure, as needed, may prevent the animal from turning the head back to bite, help with performing the examination with the other hand, and, above all, avoid inadvertent ocular prolapse.51 Guinea pigs are docile animals and may be carried by supporting its weight in one hand and cupping its dorsum with the other. Chinchillas may be generally handled in the same fashion as guinea pigs, but frequently may be removed from a cage by grasping and lifting the base of the tail, using the opposite hand to support the body.46 Prairie dogs may have a strong bond with humans or the social group. However, if untamed, they may roll quickly and bite, squirt musk from the anal sacs, or inflict significant scratches with their long, sharp nails.28 Gloves may be worn when handling prairie dogs and other aggressive rodents, but gloves limit feel and do not protect the handler against a direct bite.23,28 Gloves that protect from the bite of a laboratory rodent species may be completely ineffective with a wild rodent such as a marmot or a squirrel.19 To handle a prairie dog, a small towel may be placed over its head, to prevent biting, and then be lifted, as with guinea pigs and chinchillas. Degus should be handled in a similar manner to chinchillas, but they seem more inclined to bite. It is advised that degus be restrained with a towel or placed on a nonslip surface for examination.27 Small laboratory rodents may be transferred from cage to cage by using soft forceps; this same technique is sometimes used for small free-living rodents caught in traps.23 Plastic tubes are sometimes used for transporting smaller rodents, conducting examinations, taking radiographs, or administering anesthesia.19 Clear plastic bags may also be successfully used to restrain, measure, and sex rodents, with minimal direct handling.6 Agoutis and acouchis may be safely restrained by using a sack with a metal ring attached to one of the bag ends. This method allows the handler to take biomedical parameters while keeping the animal relatively calm.48 Most rodent species may be netted with a fine mesh, which avoids the entanglement of the claws, and larger species such as capybaras, marmots, and woodchucks may be handled by using specialized squeeze cages.19 Another option for these and other large rodents is the use of extended nets on the floor on a corridor that may be suspended to bag up the animal once it crosses through.48 Porcupines offer a unique challenge, since they have sharp quills and may move back quickly and with great force when they feel threatened. This species also has pathogenic bacteria covering the quills. Some authors recommend purpose-built restraint boxes for handling porcupines.23 Special care is required during handling of some species of rodents such as coendus (Coendu mexicana) because of their passive defensive abilities. They have modified hair (histriciformes) covered by interwoven scales acting as small tabs, and this warrants specific mechanics during handling to avoid handler injury. Two techniques have proven efficient for handling coendus, depending on the purpose for restraining. One technique requires grasping the animal by the end portion of the tail (where no quills are present), supporting the hindlimbs on a branch or stick, and exposing the hip musculature for injection. Another handling alternative is to force the coendu to enter a PVC tube, where it is immobilized to allow some procedures, including sexing, hand injections, and temperature checking.48 Wild rodents in the families Muridae and Sciuridae may be trapped by using wire cage traps and metal box traps (Sherman traps).53 Aquatic rodents such as beavers, muskrats, and nutrias may be captured in specialized hinged cage traps, called Hancock or Bailey traps, or wire cage traps set on land.53 Potential zoonotic threats (Hantavirus infection, tularemia, lymphocytic choriomeningitis virus (LCMV) infection, yersiniosis) should be considered when handling wild or feral rodents, and use of special protective gear is advised.53 In handling all rodents, care must be taken to prevent inhibition of respiration or exacerbation of stress during restraint.27,33 Nervous, distressed, sick, or debilitated rodents benefit from mild sedation for examination, diagnostics, and treatment procedures.33 Some rodents are prone to “breath holding” when physically restrained, which may cause the rodent to develop hypercapnia and bradycardia. In these species, it is best to use other methods such as an anesthetic chamber for induction.50 Basic principles of domestic mammal anesthesiology apply to exotic mammals, although the latter have specific features that must be taken into account when planning an anesthetic procedure. When performed with appropriate equipment and by experienced personnel, chemical restraint reduces stress and the risk of injury.6 According to a recent report, a higher risk of anesthesia-induced mortality exists in small exotic mammal species compared with that in dogs and cats.61 Rodents are often catecholamine-driven prey species that are more easily stressed, are commonly presented in advanced stages of disease (respiratory and nutritional-related issues) with little respiratory and cardiovascular reserve, and have anatomic challenges with regard to procedures such as endotracheal intubation and intravenous access.14 As rodents are primarily obligate nasal breathers, rodent anesthesia is sometimes complicated by upper respiratory disease such as Streptococcus infection in mice and other rodents, hypovitaminosis C and bordetellosis in guinea pigs, chlamydophilosis and adenovirus infection in several rodent species, pasteurellosis and pseudo-odontoma in prairie dogs, and mycoplamosis in rats and other rodents of the family Muridae.23,28,50 These issues may occur in association with incisor overgrowth, chronic suppurative periodontal disease, and dusty or poorly ventilated environments, all of which are frequent problems in captive rodents.28 Chemical restraint is preferable for wild-caught or rarely handled individuals if a thorough examination or prolonged procedure is necessary.6 Careful planning and preparation of the sedation or anesthesia procedure greatly contributes to success. This includes stabilizing the patient prior to the procedure (normothermic, rehydrated, good nutrition state and no metabolic derangements), calculating emergency drugs using an emergency drug chart, and preparing equipment and supplies (anesthetic drugs, catheters, fluids, anesthetic circuits according to the size of the rodent, with appropriately sized bags and masks, surgical equipment, preheated disinfectant solutions).14 Fasting is not routinely done in small rodents prior to anesthesia, since this exhausts glycogen stores and contributes to ileus in guinea pigs, chinchillas, and other herbivorous rodents.23 Also, it is important to keep in mind that many rodent species cannot vomit, so withholding food or water for prolonged periods of time before anesthesia is not required. For some rodents, food may be removed approximately 1 hour before anesthesia induction to reduce the presence of food within the oral cavity. 61 All rodents should be accurately weighed to ensure correct drug and fluid calculations.61 For all rodents, anesthetic agents that provide a rapid recovery, have specific antagonists, and are easily adjusted with regard to anesthetic depth are preferred.50 In rodent species, it is not usually feasible to administer intravenous anesthetics for induction of anesthesia. However, in zoo and pet rodents, intramuscular injections of sedatives, tranquilizers, and anesthetics are possible either by hand injection or remote delivery. Premedicating the rodent with a sedative is often recommended, since this allows for a smooth anesthetic induction. Anticholinergic drugs such as atropine and glycopyrrolate are not routinely administered to rodents as a premedication but are administered to patients that present bradycardia during the anesthetic procedure.61 Neither glycopyrrolate nor atropine influence respiration rate, core body temperature, or systolic blood pressure, alone or combined with injectable anesthetics.23,61 However, glycopyrrolate is more effective in maintaining heart rate within the normal range in rats.23 Large doses of parasympatholytics may alter GI motility in hind gut fermenters, including rodents, so the lowest doses are suggested if the use of a parasympatholytic is required.21 A balanced approach to anesthesia and analgesia must be taken for each animal, including preanesthetic analgesics, anxiolytics, local or incisional anesthetics, general anesthetics, and post-anesthesia analgesics.27 Sedatives and tranquilizers may be administered 30 to 60 minutes before induction via a face mask.50 Benzodiazepines (diazepam 0.1–5 milligrams per kilogram [mg/kg], intramuscularly, intraperitoneally, or orally [IM, IP, or PO]; midazolam 0.1–2 mg/kg, IM, IP, or subcutaneously [SC], depending on the rodent species) provide good sedation and relaxation in rodents and are useful for induction of debilitated patients (midazolam) because of the minimal cardiopulmonary effects of these agents.23 Diazepam should only be administered IV or PO, although when used IV, the patient must be monitored for hypotension caused by the propylene glycol found in most diazepam formulations.21 Because of their intractable natures and the difficulty of handling of many exotics and zoologic species, diazepam has been used IM and even SC. Erratic responses might occur when using intramuscular and subcutaneous delivery of this benzodiazepine. Midazolam, a short-acting benzodiazepine that is water-soluble, when injected subcutaneously will decrease the excitability of the rodent patient during gas induction. This drug provides adequate effective sedation for approximately 1 hour when used in rodents, and when administered IM or IV in guinea pigs and other rodents, midazolam provides proper sedation for diagnostic procedures (e.g., radiography, ultrasonography).61 It is worth mentioning that cardiorespiratory side effects associated with midazolam are minimal, so its use is not limited to healthy animals.27,61 The combination of fentanyl–fluanisone, another sedative used in guinea pigs and other small mammals, produces profound sedation and analgesia.17 Injectable anesthesia alone is a practical option for rodents when access to the head and neck is required for any procedure. In small rodents, injectable anesthesia (IP, IM, or SC) is usually administered. Ketamine is one of the anesthetic drugs most commonly used in many small and zoo rodents species, usually combined with sedatives and tranquilizers, although intramuscular injection of the former and of ketamine plus xylazine has been associated with self-mutilation in some species.23,61,43 Some rodents such as guinea pigs have limb movement at ketamine or tiletamine–zolazepam dosages expected to produce surgical anesthesia.23 Xylazine, medetomidine, and dexmedetomidine, which are α2-agonist drugs, combined with ketamine produce muscle relaxation, analgesia, and long duration of effect, and these combinations have been used for short-term immobilization and surgical anesthesia.23 Recent publications indicate that midazolam enhances the analgesic properties of dexmedetomidine in rats, so it is assumed that this benzodiazepine may be combined with other α2-agonists to provide adequate sedation and analgesia in rodents and other small mammals. α2-agonist drugs are reversed with yohimbine, tolazoline, or atipamezole.23 Nonetheless, many rodents presented to veterinarians are clinically ill, geriatric, and therefore potent α2-agonists such as dexmedetomidine are only used in young healthy individuals.4,14 Ketamine–benzodiazepine combinations produce less cardiopulmonary depression and analgesia but good muscle relaxation.23 Opioids (butorphanol, buprenorphine) and other analgesic drugs may be added to this combination to promote adequate analgesia in rodents. The tiletamine–zolazepam combination has been used in many laboratory, pet, and zoo rodent species, with variable results, for minor surgery, diagnostic procedures, and routine preventive medicine programs.23,43 The author of this chapter has used the tiletamine–zolazepam combination at published doses (4–6 mg/kg, IM) in beavers, porcupines, and capybaras for routine health checks and has combined it with opioids (butorphanol 0.1 mg/kg, IM) for wound cleaning and teeth checking or trimming. It has been also used as induction for longer procedures in zoo settings, where inhalant anesthesia has been used subsequently for maintenance. The combination of medetomidine–midazolam–fentanyl has been used successfully in chinchillas, guinea pigs, rats, mice, and hamsters, and the main advantage of this combination is that it is completely reversible with flumazenil, atipamezole, and naloxone.35 Midazolam combined with fentanyl–fluanisone produces neurolepto-analgesia with adequate skeletal muscle relaxation in mice, rats, gerbils, hamsters, and guinea pigs.4,61 Parenteral anesthesia is used in rodents when inhalant anesthesia is not available, when handling is stressful or difficult for some zoo rodents, for immobilization of rodents in the wild, to reduce inhalant anesthetic pollution, to enhance analgesia (preemptive analgesia) and for other purposes convenient to the handler or the facility.23 Propofol, a hypnotic agent, is used in rodents and other species for induction and maintenance of anesthesia when administered as a continuous rate infusion (CRI), manually controlled infusion (MCI), and total intravenous infusion (TIVA) by intermittent bolus. This drug may be diluted with saline for injection in small rodent species. It has a higher elimination clearance and a shorter elimination half-life compared with other injectable agents, and its clearance rate is faster than the liver blood flow.18,23 Propofol-induced apnea is related to dose, rate of injection, and the concurrent administration of other drugs. Propofol is a poor analgesic.23 Etomidate is a nonbarbiturate that is an injectable, potent, short-acting, hypnotic, and anesthetic agent. It has been used at a rate of 1 mg/kg in rodents (prairie dogs, chinchillas, guinea pigs) by intravenous delivery for teeth inspection or trimming,28 so potentially it might be used in other zoo rodent species for the same purposes. Epidural anesthesia was performed in seven agoutis (Dasyprocta azarae) with the use of lidocaine (5 mg/kg) in the lumbosacral space 5 minutes after induction with azaperone, meperidine, xilazine, and ketamine. Duration of analgesia was 80.86 ± 16.1 minutes, and no complications from the epidural technique were observed.55 Using reversible injectable agents and combinations provides better control of anesthetic depth, less hypothermia, less cardiopulmonary depression, and shorter recovery times.21 Inhalant anesthesia is the primary component of most clinical anesthetic regimes, and so is the preferred method for chemical restraint of rodents, since it has the advantage of providing oxygen, resulting in a relatively rapid and smooth induction and recovery, and allows safe and fast titration of the anesthetic dose to the required effect.6,23 Preoxygenation (either with an oxygen chamber or an induction chamber) of rodent patients, especially in those undergoing respiratory or cardiac diseases, improves circulatory and tissue oxygen reserves and saturation.35 Non-rebreathing anesthetic circuits (e.g., Bain circuit or Ayre’s T-piece) have low dead space and low resistance and so are recommended for use in small rodents.61 Intubation is not easily possible in many species of rodents, so the anesthetic is often administered into a chamber (for small- to medium-sized rodents) or through a tight-fitting mask connected to the breathing system.23,35 Small- and medium-sized rodents are preferably induced and maintained with an inhalant anesthetic. Rodents such as prairie dogs, chinchillas, squirrels, African pouched rats, maras, and other similar-sized species have been intubated with a 2- or 2.5-mm uncuffed endotracheal tube, with the use of a “blind” technique or with the aid of an otoscope, laryngoscope, or endoscope.23,28 Endoscopy provides the best visualization of the epiglottis and minimizes trauma during tube placement.21 Endotracheal tubes are commercially available from the smallest 1-mm internal diameter (uncuffed) to 3-mm (cuffed) and bigger. Actually, for the smallest rodent patients, endotracheal tubes are made from over-the-needle catheters (14 g and bigger) or urinary catheters.23 In capybaras, endotracheal tubes of 6-mm internal diameter have been used. The rodent soft palate is fused to the base of the tongue, and entry to the glottis is through the small opening of the palatal ostium, a structure present in chinchillas, guinea pigs, and capybaras.23 This structure is highly vascular and easily traumatized.21 Laryngeal masks have been used successfully in rabbits weighing more than 3 kg and so could potentially be useful in similar-sized rodents. This type of mask must be easily inserted and provide a good fit for an adequate seal.61 The recommended inhalation agents for rodents are isoflurane and sevoflurane, in a suggested concentration of 3% to 5% for induction and 1.5% to 3% for maintenance of anesthesia for isoflurane, and concentrations of 5% to 6% are recommended for induction when sevoflurane is used. Those levels of gas anesthesia vary, depending on other drugs used prior to the inhaled agent or if any other was used at all. Isoflurane produces a dose-dependent cardiopulmonary depression, is a poor analgesic, and has the advantage of not sensitizing the myocardium to catecholamine-induced arrhythmias.23 Sevoflurane is similar to isoflurane, but it produces more rapid induction and recovery, and it is less irritating to inhalation. Guinea pigs are particularly prone to apnea with the use of inhalant anesthetics and require careful monitoring and sometimes the use of a respiratory stimulant (e.g., doxapram at a rate of 5–10 mg/kg, IV or IM) to keep respirations regular.27 The small size of rodents makes venous and intraosseous catheterization difficult. Some potential catheterization sites include the cephalic, saphenous, and auricular veins.23 Intraosseous cannulation may be useful in smaller patients or during cardiovascular collapse. Common sites for intraosseous cannula placement are the trochanteric fossa of the femur, the tibial crest, and the humerus. Depending on the rodent size, products that may be used as intraosseous cannulas include 18-to-24-gauge 1-to- For all rodent species, preemptive analgesia and sedation are critically important. High levels of circulating catecholamines, combined with the stress of handling or restraint, hypoxemia, hypercarbia, and unpredictable responses to anesthetic agents may lead to respiratory and cardiac arrest.14 The two main groups of analgesic premedication—opioids and nonsteroidal anti-inflammatory drugs (NSAIDs)—are combined or used alone in rodents and other species.23 An effective pain management plan should include drugs of different classes and a multimodal approach. Midazolam may also be combined with opioid compounds such as butorphanol to provide additional analgesia.61 Analgesic medications administered IV as a CRI may be titrated to effect, potentially reducing other drug doses within the anesthetic protocol. Microdose ketamine via intravenous CRI may be an effective analgesic.21,23 Opioid drugs are effective analgesic agents for the control of acute or chronic visceral pain. In small exotic mammals, including rodents, butorphanol, buprenorphine, and fentanyl are regularly used and well tolerated. The main adverse side effects of opioids in small mammals are drowsiness and respiratory depression, which is dose dependent.35,61 Pica and gastric distension have been observed in rats receiving high doses of buprenorphine (0.5 mg/kg, SC).12 Opioids may also be used for CRI. When used as a low-dose intravenous CRI, opioids do not induce GI stasis in small hind gut fermenters and so may be a part of balanced anesthesia and analgesia protocols. Remifentanil is an ultra-short-acting µ-agonist opioid that is very suitable for CRI in small mammals. Butorphanol has also been used as a CRI, although no published data on small exotic mammals are available.21 Tramadol, a synthetic opioid drug, is a centrally acting drug, being both a weak opioid agonist with selectivity for the µ-receptor, and a weak inhibitor of the reuptake of norepinephrine and serotonin. A few studies have been performed in rodents, mainly in laboratory animals. A study in rats showed that tramadol administered intraperitoneally (1–25 mg/kg) showed delayed responses after thermal or ischemic noxious stimuli, and subcutaneously at the site that received noxious stimuli provided local analgesia. The same study found that tramadol and gabapentin worked synergistically to provide analgesia in rats.56 The most common NSAIDs drugs used in rodents include meloxicam, carprofen, and ketoprofen. Clinical experience indicates that doses <1 to 2 mg/kg of meloxicam in rats do not adequately alleviate pain after surgery.61 Local anesthesia and regional (epidural) anesthesia have proven efficacious in providing good analgesia in rodents for minor procedures such as cesarian section in guinea pigs. Lidocaine and bupivacaine are commonly used local anesthetics that may be applied topically, via tissue infiltration, intra-articularly, as regional nerve block, or by epidural injection.23,61 Important nerve blocks for dental surgery in rodents include infraorbital, mental, mandibular, and maxillary nerve blocks. Other sites for nerve blocking in rodents and small mammals are the brachial plexus, the sciatic nerve, and the intratesticular nerve.21 Volumes of local anesthetic agents used for epidural injection vary between 0.1 and 0.2 milliliters per kilogram (mL/kg).61 It is essential to record supportive care and monitoring minute-by-minute in rodents. Eyes should be well lubricated to prevent corneal desiccation and ulceration. The order of loss of reflexes in rodents is as follows: palpebral, pedal, jaw tone, ear pinch reflex (surgical plane).62 A fixed, dilated pupil that is unresponsive to light is a cross-species indicator of excessive depth.23 Monitoring of anesthesia requires recording heart rate, respiratory rate, body temperature, and analgesia. Heart rate varies widely among rodent species and is determined by temperature, size, metabolism, respiratory rate, and the presence or absence of painful stimuli.21 Quality pediatric stethoscopes, instead of esophageal stethoscopes, are recommended for small species of rodents. Because of the rapid heart rate of many small rodents, electrocardiography (ECG) complexes are assessed with sweep speeds of 100 millimeters per second (mm/s) and 200 mm/s.21 The Doppler flow detector is used where major arteries are close to the skin, for example, the ventral aspect of the tail base; the carotid, femoral, and auricular arteries; or directly over the heart. Normal systolic blood pressure measurements obtained with the Doppler range from 90 to 120 millimeters of mercury (mm Hg).21 Some authors have successfully measured blood pressure in the hindlimbs of guinea pigs, chinchillas, degus, prairie dogs, and other rodents by using a size 1 cuff with sphygmomanometer and audio Doppler ultrasonography just above the medial tarsus.27 As in other species, the respiratory rate and character should be monitored closely. In addition to direct monitoring of respiration, respiratory monitors are indicated in monitoring rodent anesthesia protocols, but those that are triggered by a thermistor in the airway function well only in patients weighing more than 500 g. To assess ventilation, end-tidal carbon dioxide (PET-CO2) monitoring, using a side-stream capnograph, is useful. Pulse oximetry (SpO2) is used in many species to evaluate arterial hemoglobin oxygen saturation. It has been evaluated in rats but appears accurate at hemoglobin saturation levels greater than 70%.23,35 Pulse oximeters may be used on the ears or tongue of guinea pigs, chinchillas, prairie dogs, and other medium-sized rodents, on the feet of most rodent species, and also on the tail, or vaginal or rectal mucous membranes.21,35 Some sick or debilitated animals require intermittent positive-pressure ventilation (IPPV) during anesthesia, which, in the case of rodents, is recommended at initial inspiratory pressures of 5 to 10 centimeters of water (cm H2O) and rates of 6 to 10 breaths per minute.21 To reduce the morbidity and mortality associated with anesthetic procedures in rodents, it is of utmost importance to monitor core body temperature. Because of the ratio of large surface area to volume, hypothermia is one of the most frequent complications in anesthetized rodents. An electronic thermometer probe may be fastened to the perineal area to continuously monitor the body temperature.23,27 Small rodents (less than 500 g) require an ambient temperature of 35° C to 37° C during recovery and larger ones between 25° C and 35° C.50 It is recommended that rodents be wrapped in aluminum foil or bubble wrap immediately after induction. Supplemental heating (electric heat pads, heated operating tables, forced warm air blankets, heat lamps, hot water bottles, and towels) should be provided during and after anesthesia. It is of utmost importance to stress that heating devices should not be in direct contact with the animal’s skin or body surface to avoid iatrogenic burns.35,50 Once the anesthetic procedure has been completed, the animal is placed in a warm, quiet environment and monitored. Monitoring of body functions must continue until the patient is fully alert, since general anesthesia may have adverse effects on gut motility and flora, especially in herbivorous rodents.27 It is recommended that GI function be assessed, and if necessary, administration of gut motility enhancers (cisapride, metoclopramide, or ranitidine) is advised. Prolonged recovery is usually caused by hypothermia, hypoglycemia, and anesthetic overdose, altered drug elimination, or all of these factors. Assisted feeding with the appropriate nutritional products (herbivores, omnivores, and carnivores) is of great importance during recovery in rodents. Ongoing fluid therapy may also be necessary until the animal is able to maintain hydration. Table 42-4 details chemical agents commonly used as sedatives, anesthetics, and analgesics in rodents. TABLE 42-4 Sedatives, Anesthetics, and Analgesics Commonly Used in Rodents (doses are given in mg/kg)
Rodentia
Biology
Classification
Geographic Distribution
Unique Anatomy
Physiology
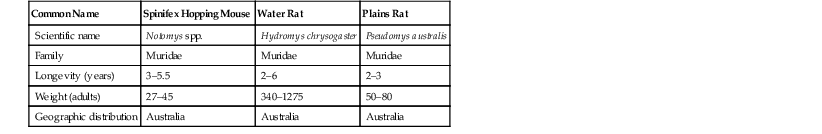
Common Name
Spinifex Hopping Mouse
Water Rat
Plains Rat
Scientific name
Notomys spp.
Hydromys chrysogaster
Pseudomys australis
Family
Muridae
Muridae
Muridae
Longevity (years)
3–5.5
2–6
2–3
Weight (adults)
27–45
340–1275
50–80
Geographic distribution
Australia
Australia
Australia
Special Housing Requirements
Feeding
Diets of Free-Ranging Rodents
Diets of Captive Rodents
Unique Nutritional Requirements
Restraint and Handling
Chemical Restraint
Anesthesia and Surgery
 -in. spinal needles or 18- to 25-gauge 1-inch hypodermic needles.21
-in. spinal needles or 18- to 25-gauge 1-inch hypodermic needles.21
Drug Name
Mouse/Gerbil, Hamster
Rat
Guinea Pig, Prairie Dog, and Chinchilla
Degu
Duprasi
Beaver, Capybara, and Porcupine
Native Australian Rodents
Comments
OPIOIDS
Buprenorphine
0.05–0.1, SC, q6-12h
0.05–0.1, SC, IM, q6-12h
0.01–0.05, SC, IM, IV, q6-12h
0.05–0.1, SC, q8-12h
0.05–0.1, SC, q1 h
0.01–0.03, IM, SC, q8-12h
—
Can be associated with pica in rats
Butorphanol
1–2, SC, IP, q2-4h
1–2, SC, IM, IV, q2-4h
0.5–1.0, IM, IV, q4h (GP) 0.2–2.0, IM, IV, q2-4h (Ch)
0.4–2.0, IM, q8h
1–5, SC, q4h
0.5, IM, SC, q4h
—
Weaker analgesic than buprenorphine
Hydromorphone
0.2–0.4, SC, q6h
0.2–0.5, SC, q6-8h
0.2–0.5, SC, IM, q6-8h
—
—
—
—
—
Meperidine
10–20, SC, q2-4h
10–20, IM, SC, q2-4h
10–20, IM, SC, q2-4h
10–20, SC, IM
20, SC, IM, q2-3h
—
—
—
Morphine
2–5, SC, IM, q4h
0.5–5, SC, IM, q4h
2–5, SC, IM, q4h
2–5, IM, q4h
2–5, SC, q2-4h
1–3, IM, SC, q4-6h
—
Most commonly used as a single dose preoperatively
Nalbuphine hydrochloride
2–4, SC, IM, q2–4h
1–2, SC, IM, q2-4h
1–2, IM, q2-4h
—
—
—
—
—
Oxymorphone
0.2–0.4, SC, q6-12h
0.2–1.5, SC, q6-12h
0.2–0.5, SC, IM, q6-8h
0.2–0.5, SC, IM, q8-12h
0.2–0.5, SC, IM, q8-12h
—
—
—
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
Carprofen
5–10, SC, PO, q12-24h
1–5, SC, PO, q12-24h
1–5, PO, q24h
4, PO, q24h
5, PO, q24h
—
—
—
Flunixin
2.5, SC, q12h
2.5, IM, SC, q12h
1–2, SC, q24
1–2, SC, q24
2–5, SC, q12-24h
0.5, IM, SC, q12-24h
—
Not recommended at least in smaller rodents due to potential renal damage
Ketoprofeno
2–5, SC, IM, q12-24h
2–5, SC, IM, q12-24h
1–2, SC, IM, q12-24h
—
—
1–3, IM, SC, q24h
—
—
Meloxicam
1–5, PO, SC, q24h
0.5–2.0, PO, SC, q24h
0.1–0.3, PO, SC, q24h
0.2–0.4, SC, PO, q24h
0.2–0.4, SC, PO, q24h
0.1–0.3, SC, PO, q12-24h
—
—
Paracetamol
—
—
1–2 mg/mL in drinking water
1–2 mg/mL in drinking water
1–2 mg/mL in drinking water
—
—
—
OTHER ANALGESICS
Tramadol
5–40, IP, q12-24h (M)
5–10, PO, q12-24h (H, G)
5–20, PO, SC,
IP q12-24h
5-40, SC
5–10, PO, q12-24h
—
—
—
—
—
Gabapentin
50, PO, q24h (H)
—
3–5, PO, q12-24h
—
—
—
—
—
ANESTHETICS
Ketamine/Acepromazine
50–150/2.5-5.0, IP
50–150/2.5–5.0, IP
20–50/0.5–1.0, IP, IV
—
—
—
—
—
Ketamine/Diazepam
50–100/2–5, IP
40–100/3-5, IP
20–40/1–5, IM
20–30/1–2, IM
Not published
—
—
Poor analgesia
Ketamine/Dexmedetomidine
—
—
40/0.5, IM, IP
—
50/0.5, IP
—
—
—
Ketamine/Medetomidine
50–100/0.1–0.5, IP, IV
45–75/0.3–0.5, IM, IP
5–40/ 0.05–0.5
—
—
3–4/0.03–0.04, IM, IV
—
Lowest doses of ketamine for chinchillas
Ketamine/Midazolam
—
5–10/0.25–0.5, IP
5–10/0.5–1.0, IM
—
—
3–4/0.03–0.04, IM, IV
—
—
Ketamine/Xylazine
35–200/2–10, IP
40–100/5–10, IM, IP
20–40/1–2, IM
40/2–4, IM
50/5, IP
5–10/1–2, IM
—
—
Tiletamine/Zolazepam
50–80, IP
5–10, IP, IM
20–40, IM, for chinchillas
—
—
4–6, IM
20–40, IM
Australian brown rat
Propofol
7.5–26, IV
2–5, IV
3–5, IV
—
—
6–8, IV
—
—
REVERSAL AGENTS
Atipamezole
≥1, SC, IM, IV
0.1–1, SC, IM, IV, IP
≥1, SC, IM, IV
1, SC, IM, IV, IP
1, SC, IM, IV, IP
—
—
1 : 1 volume reversal of medetomidine or dexmedetomidine
Flumazenil
—
0.1–1.0, IM, IV, IP
0.05–0.1, SC, IM, IV
0.1, SC
—
—
—
Reversal of benzodiazepines
Naloxone
0.01–0.1, SC, IP
0.01–0.1, SC, IP
0.01–0.1, SC, IP
0.01–0.1, SC, IP
0.01–0.1, SC, IP
—
—
Narcotic reversal
Yohimbine
0.5–1.0, IM, IV
0.2, IV; 0.5 IM
0.5–1.0, IV; 2, IM
2, IM
2, IM
—
—
Reversal for xylazine
LOCAL ANESTHETICS
Bupivacaine (local and regional blocks)
<2
<2
<2
—
—
—
—
Dosages for local blocks may extend to 2 mg/kg as the maximum dose
Lidocaine (local and regional blocks)
<4
<4
<4
—
—
—
—
Short duration of 20–30 minutes in rats
PREANESTHETICS
Atropine
0.04 mg/kg, SC, IM
0.05 mg/kg, SC, IP IM, IV
0.05 mg/kg, SC, IM, IV
0.05–0.1 mg/kg, SC, IM
0.1–0.4 mg/kg, SC, IM
0.03 mg/kg, SC, IM
—
Lower doses for herbivores
Glycopyrrolate
0.02–0.5 mg/kg, SC, IM
0.02–0.5 mg/kg, SC, IM, IV
0.01–0.02 mg/kg, SC, IM IV
0.01–0.02 mg/kg, SC, IM, IV
0.01–0.02 mg/kg, SC, IM, IV
0.01 mg/kg, SC, IM
—
Lower doses for herbivores
SEDATIVES AND TRANQUILIZERS
Acepromazine
0.5–1.0 mg/kg, IM, IP
0.5–1.0 mg/kg, IM, SC
0.5–2.5 mg/kg, IM, SC
0.5–1.0 mg/kg, IM, SC
0.5–1.0 mg/kg, IM, SC
0.1 mg/kg IM
—
May induce seizures in gerbils
Diazepam
3–5 mg/kg, IP, PO
3–5 mg/kg, IP, PO
1.0–2.5 mg/kg, IM, IP, PO
0.5–3.0 mg/kg, IM
3–5 mg/kg, IM
0.1–1.0 mg/kg, IM, IP, PO
—
Erratic results and irritation if given IM
Midazolam
2–3 mg/kg, IM, SC
2.5 mg/kg, SC, IM
0.4–2.0 mg/kg, IM
0.4–2.0 mg/kg, IM
1–2 mg/kg, IM
0.1–0.5 mg/kg, IM
—
Lower doses for premedication
Fentanyl/Fluanisone
*Hypnorm
0.2–1 mL/kg, IM
0.2–3.0 mL/kg, IM
0.5–1.0 mL/kg, IM
—
—
—
—
—
Medetomidine
0.03–0.2 mg/kg, SC
0.08–0.2 mg/kg, IM
0.1–0.5 mg/kg, IM, SC
—
—
—
—
Variable effects in guinea pigs
Dexmedetomidine
0.015–0.5 mg/kg, SC, IP
0.015–0.5 mg/kg, SC
0.05 mg/kg, SC, variable effects
—
0.1–0.2, mg/kg, SC
—
—
Half the dose of medetomidine
Xylazine
10–15 mg/kg, IP
10–15 mg/kg, IP
2–10 mg/kg, IM, IP
Not used by itself
Not used by itself
1–5 mg/kg, IM, SC
—
Used more for zoo rodent protocols ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rodentia
Chapter 42