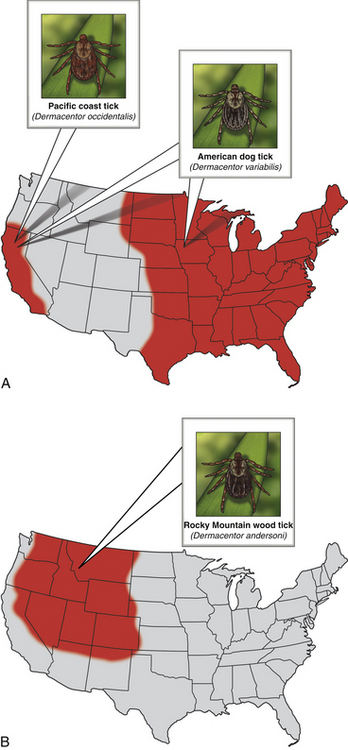
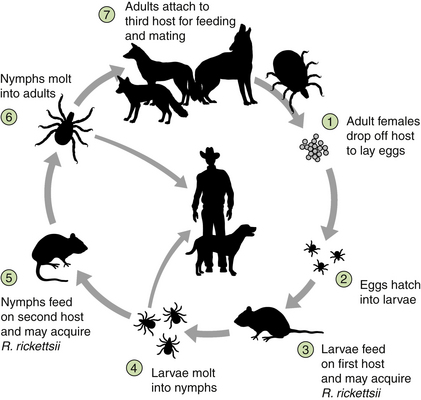
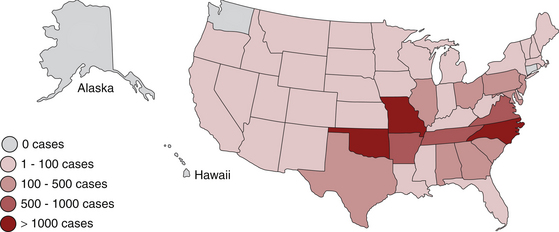
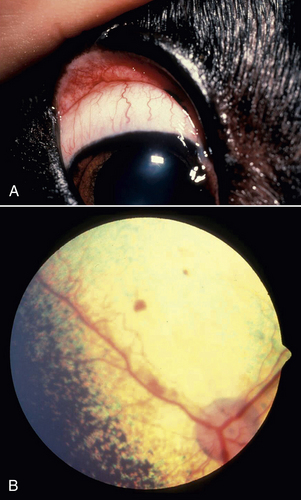
Chapter 30 Rocky Mountain spotted fever (RMSF) is caused by Rickettsia rickettsii, an obligately intracellular bacteria in the alphaproteobacteria (genus Rickettsia, family Rickettsiaceae, order Rickettsiales).2 The terminology used to describe rickettsial disease is confusing and inconsistent due to multiple changes in the taxonomic classification of organisms in recent years.3 The terms “rickettsial disease,” “rickettsioses,” and even the term “Rickettsia” have been used to refer to several obligately intracellular organisms including Rickettsia, Bartonella, Ehrlichia, Anaplasma, Coxiella, and Neorickettsia. Previously, all of these organisms belonged to the order Rickettsiales and most were in the family Rickettsiaceae, based on their fastidious or intracellular nature and other characteristics. Therefore, collectively members of these diverse genera were referred to as “rickettsial organisms.”4,5 Recent advances in molecular biologic techniques have resulted in the reclassification of several of these organisms. Many have been moved out of the family Rickettsiaceae, and others have been moved out of the order Rickettsiales. Now the order Rickettsiales includes only two families, the family Anaplasmataceae, which contains the genera Anaplasma, Ehrlichia, Wolbachia, and Neorickettsia; and the family Rickettsieaceae, which includes the genera Rickettsia and Orientia.2,3,5 Currently, “rickettsial” refers to diseases caused by organisms in the genera Anaplasma, Ehrlichia, Rickettsia, Neorickettsia, and Orientia, “rickettsioses” refers to diseases caused by organisms in the family Rickettsiaceae (Rickettsia and Orientia), and the term “Rickettsia” refers specifically to members of the genus Rickettsia.3–5 The genus Rickettsia is currently divided into the spotted fever group (SFG) and the typhus group based on phenotypic and, more recently, genotypic characteristics.3,5 Some of the phenotypic characteristics that have historically been used to group the organisms include the types of vectors that transmit them and the pathophysiologic manifestations of the disease.3 The SFG rickettsial species are transmitted by arthropod (primarily tick) vectors and infect endothelial cells in mammalian hosts.5 The two most pathogenic and well-studied SFG rickettsiae are Rickettsia rickettsii, the cause of RMSF in the Western Hemisphere, and Rickettsia conorii, the cause of Mediterranean spotted fever (MSF) in other areas of the world. The first case of RMSF was described in the late 1800s and the first case of MSF was described in the 1920s.6 Therefore, these organisms and their associated diseases are the most well characterized among the SFG rickettsiae.3 Currently, there are 20 or more species of SFG rickettsiae.3,6 Some species appear to be nonpathogenic endosymbionts of ticks.6 However, some SFG rickettsiae previously thought to be nonpathogenic, such as R. parkerii and R. massiliae, have recently been associated with disease in people.7–9 Previously thought to have rather limited geographic boundaries, many SFG rickettsiae have also been detected in expanding geographic locales around the world.6 For example, RMSF, originally described in the Bitterroot Valley of Montana, was subsequently found to be a frequent tick-transmitted infection in the eastern United States, but only recently has transmission via the brown dog tick been documented in the southwestern United States.10,11 Increasing travel and the effects of climate change on tick populations and habitat are thought to be in part responsible for this phenomenon.12,13 Hosts, Life Cycle, and Transmission Dogs are sentinels for SFG rickettsioses in people. Both R. rickettsii and R. conorii infect and cause disease in dogs.14–20 Rickettsia rickettsii infection in dogs can occur before, or coincide with, outbreaks of RMSF in people in the same household or community.21–24 Several serosurveys in endemic areas have shown an increased risk of MSF in people who live near dogs that are seropositive for SFG rickettsiae.25–27 Other SFG rickettsial species likely infect dogs, but the extent to which they cause clinical disease has not yet been established. Species implicated in natural infection in dogs include R. massiliae, R. japonica, and R. australis.28–31 Young and purebred dogs are overrepresented in some but not other studies.17,18 Although some studies suggest male dogs are at increased risk, no sex predilection has been definitively documented.17,18,32 Severe disease has been reported in English springer spaniels with phosphofructokinase (PFK) deficiency and German shepherd dogs.18,32 Although antibodies to SFG rickettsiae can be detected in cats that live in endemic areas, the ability of SFG rickettsiae to actively infect and cause disease in cats has not been well characterized.33 Similarly, the ability of typhus group Rickettsia to cause disease both in dogs and cats has also not been well characterized and will not be discussed here. Rickettsia rickettsii is transmitted by several hard (ixodid) ticks including Dermacentor variabilis (the American dog tick) (Figure 30-1, A), Dermacentor andersoni (the Rocky Mountain wood tick) (Figure 30-1, B), Amblyomma americanum, Amblyomma cajennense, Amblyomma aureolatum, and Rhipicephalus sanguineus.34–38 Amblyomma cajennense and to a lesser extent, A. aureolatum transmit the rickettsia in South America. Ticks that transmit R. rickettsii feed once during each life stage (Figure 30-2). Both transstadial and transovarial transmission occurs in Dermacentor spp. infected with R. rickettsii, so these ticks serve as a reservoir of infection.39,40 Dermacentor spp. and Amblyomma spp. are three-host ticks. Infection is transmitted among mammalian hosts and ticks when the tick feeds on different hosts during each stage of molting. The sylvatic cycle for Dermacentor ticks involves small mammals, such as chipmunks, pine voles, mice, and ground squirrels. After organisms are ingested by the tick, SFG rickettsiae initially replicate in the epithelial cells of the tick midgut, enter the hemolymph and hemocytes, and then multiply in tissues.40 Once in the salivary glands, the organism is transmitted to a naïve mammalian host on feeding. Transmission can occur within hours of attachment. However, this may be prolonged (up to 48 hours) when changes in virulence occur under conditions such as starvation of the tick. These “dormant” rickettsiae undergo a process called reactivation after the tick begins feeding.41 Therefore, rapid tick removal can decrease the risk of transmission in many, but not all, circumstances. FIGURE 30-1 A, Distribution of Dermacentor variabilis and Dermacentor occidentalis ticks in the United States. B, Distribution of Dermacentor andersoni ticks in the United States. FIGURE 30-2 Transmission cycle of Rickettsia rickettsii in tick populations, people, and dogs. After adult female ticks lay eggs and they hatch (1 and 2), Dermacentor spp. ticks acquire R. rickettsii upon feeding as larvae or nymphs on rodents (3); they then transmit during subsequent feeding as nymphs or adults (4 and 6). Adult ticks feed on a variety of canid species, such as foxes and possibly wolves and coyotes. In addition, R. rickettsii infection can be maintained transovarially in Dermacentor ticks, so some larvae that hatch from the egg mass already harbor an infection. (Redrawn from Nicholson WL, Allen KE, McQuiston JH, et al. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010;26[4]:205-212.) Rhipicephalus sanguineus is a one-host tick, with dogs being the preferred host.42 Rh. sanguineus can adapt to hot environments and commonly resides in walls of housing structures in close proximity to humans.42 It occasionally feeds on humans, especially when ambient temperatures are high.12 The role of this tick as a reservoir for R. rickettsii in nature has yet to be elucidated. The role of dogs as a natural reservoir of infection for R. rickettsii is also unknown.39 Dogs are thought to act as incidental hosts when Dermacentor ticks are the vectors, because ticks rarely acquire the organism from rickettsemic dogs.43 In contrast, all stages of Rh. sanguineus acquired infection at a high rate from experimentally infected dogs in one study, but this may have been related to the virulence of the rickettsial strain used.38 A relatively high infection rate with SFG rickettsiae in naturally infected Rh. sanguineus has also been described.37,44 Therefore, dogs may play a role in maintenance of a reservoir of R. rickettsii infection in endemic foci where Rh. sanguineus is the primary vector. Rocky Mountain spotted fever occurs in North, Central, and South America (where it is known as Brazilian spotted fever). In North America, most cases of RMSF occur in the southeastern and south central states (Figure 30-3). Disease distribution in the United States primarily follows the distribution of the primary vectors, D. variabilis and D. andersoni, although the importance of D. variabilis as vectors in endemic areas has recently been questioned.45 Although disease can occur any time of year, most cases of canine and human RMSF are reported from April through October, months of peak tick activity.17,46,47 Dogs that live outdoors, particularly those with access to shrubs and high grass, are at increased risk.17 FIGURE 30-3 Geographic distribution of the incidence of spotted fever group rickettsioses in people in the United States, 2005-2009. (Compiled from data from the Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Reports.) In recent years the incidence of RMSF in people has increased.47 Some of the increase may be due to misdiagnosis of disease caused by species of SFG rickettsiae other than R. rickettsii.48,49 A similar increase in seroprevalence rates has been documented in dogs suspected to have a tick-borne infection in the United States.50 The geographic distribution of RMSF in the United States has also increased beyond the distribution of D. variabilis and D. andersoni. Rhipicephalus sanguineus is the main vector for R. rickettsii in some parts of the Western Hemisphere, including Mexico and now the southwestern United States. In South America, Amblyomma species are the main tick vector. Tick species other than D. variabilis, D. andersoni, and Rh. sanguineus are also infected with R. rickettsii in the United States.44 For example, A. americanum transmitted RMSF to a person in North Carolina.36 An outbreak in Mexicali, Mexico, was attributed to R. rickettsii transmitted by Rh. sanguineus ticks associated with dogs.51 This prompted ongoing surveillance efforts in ticks, dogs, and people in southern California, a nonendemic area for RMSF. Thus far, no R. rickettsii has been found in Rh. sanguineus ticks from dogs residing in animal shelters located just across the U.S. border from the outbreak.52,53 This may be due to subtle differences in microenvironment or other factors that affect regional infection rates in ticks.54 The endotheliotropism associated with these bacteria results in the characteristic clinical sign of infection, which is disseminated vasculitis. In people, cutaneous macules, papules, and petechiation that occur in association with vasculitis form a rash that looks like “spots,” hence the name Rocky Mountain spotted fever.6 The clinical signs, time course of illness, and response to therapy for RMSF in dogs are very similar, if not identical, to those associated with RMSF in people.17–20,34 However, cutaneous lesions are not always present in dogs or people (“Rocky Mountain spotless fever”). In people, spotted fever rickettsioses caused by organisms other than R. rickettsii are often associated with eschar (an area of cutaneous necrosis) formation.2,13 Clinicians should keep in mind that dogs from nonendemic areas that have classic signs of RMSF, or those that present with atypical clinical signs, may be infected with R. rickettsii or other SFG rickettsial species. Once the organism is inoculated, it spreads through lymphatics or directly into the bloodstream to the small capillaries. R. rickettsii primarily infects endothelial cells, although smooth muscles and monocytes may also be infected.2,55 The bacterial outer membrane protein A (OmpA) and outer membrane protein B (OmpB) are important for attachment, adhesion, and virulence.56,57 These proteins are also responsible for differences in serotype, and antibodies to OmpB confer immunity to infection in experimental settings. SFG rickettsiae enter cells by inducing phagocytosis and are released into the cytoplasm through the action of enzymes such as phospholipase D and hemolysin C.57 The bacteria live in the cytoplasm and the nucleus, deriving nutrients and energy from the host cell.2,56 They spread from cell to cell by inducing actin to polymerize, which pushes the bacteria directly into adjacent cells.57,58 This helps them evade the immune response and to disseminate without rupturing the cell. They are also released into circulation when they exit the luminal surface of the cell membrane or when endothelial cell death or detachment occurs.57 Spotted fever group rickettsiae activate the transcription factor NFκB, which inhibits apoptosis and fosters further growth of the organism.56,57 Damage to endothelial cells leads to vasculitis and an increase in microvascular permeability. Mechanisms of cellular damage include oxidative injury through the production of reactive oxygen species, cellular necrosis, and induction of endothelial apoptosis by CD8+ T cells.55–57,59 Activation of PFK may be important in maintaining vascular integrity and energy metabolism in endothelial cells under hypoxic or oxidative stress,60,61 which may explain the predisposition of English springer spaniels with PFK deficiency to severe disease. Vasculitis associated with R. rickettsii infection manifests as disordered primary hemostasis, tissue edema, hypovolemia, and microthrombosis. Increased vascular permeability and the associated edema and hypovolemia results from disruption of adherens junctions, endothelial cell death, expression of inflammatory cytokines such as Il1-β, IFN-γ, and TNF-α, and induction of COX-2 with subsequent prostaglandin production.55 Microthrombosis results from altered platelet adherence to endothelium, increased tissue factor expression, increased plasminogen activator inhibitor, and the release of von Willebrand’s factor.55 Low numbers of organisms circulate in blood for approximately 13 days after infection, which includes the time that clinical signs are observed.19,62,63 Organisms are free and also contained within circulating endothelial cells, which are thought to be released from the vessels because of decreased adhesion after rickettsial invasion.57 Thus, RMSF is an acute disease. Chronic infection has not been documented in dogs or people. Co-infection with other vector-borne agents is common and should be considered if the clinical signs are atypical, if there is an incomplete response to doxycycline therapy, or if clinical signs have been present for a week before the time of evaluation.17,64 Because of variation in the extent and severity of vascular injury among dogs, a range of signs can occur, and importantly, disease manifestations are initially mild and nonspecific.15,17,18 Often, there is no known history of a tick bite. Therefore, the clinician (physician and veterinarian) must have a high index of suspicion in order to correctly diagnose and treat this disease. This is very important because a delay in diagnosis and appropriate antimicrobial therapy dramatically increases morbidity and mortality in people and in dogs.17,46 A “One Health” approach to the management of canine and human RMSF is clearly logical. Lethargy and anorexia are common and may be the only clinical signs. Vomiting and diarrhea occur frequently in dogs and people with RMSF. Melena may be observed, as may a variety of CNS abnormalities including vestibular disease and seizures.17,18,20 Dramatic and rapid weight loss has been described.20 Fever is present in approximately 80% of naturally infected dogs. Ocular signs are also frequently observed and may include a mucopurulent discharge, scleral and conjunctival injection and hemorrhage, conjunctivitis, uveitis, retinal hemorrhage, and retinitis (Figure 30-4). Lymphadenomegaly and splenomegaly also occur. Respiratory abnormalities include nasal discharge, epistaxis, and tachypnea. Mucocutaneous and cutaneous abnormalities include petechiae, ecchymosis, peripheral edema (which can be localized over a joint, the prepuce, or the mammary chain), hyperemia, and necrosis. Gangrenous necrosis can be so severe as to require reconstructive surgery after successful treatment of the acute febrile illness.15–18,20,65,66 Orchitis and scrotal edema, hyperemia, and epididymal pain are common in intact male dogs and should prompt consideration of RMSF when present. Generalized myalgia and arthralgia can be observed. Arrhythmias may also be detected. CNS abnormalities can be focal or generalized and include paraparesis, tetraparesis, ataxia, hyperesthesia, ataxia, central or peripheral vestibular signs, stupor, seizures, and/or coma. Neurologic signs are more common in dogs with a high R. rickettsii antibody titer, which suggests a longer duration of illness or a delay in diagnosis.17 Residual neurologic deficits may occur after infection in severely affected individuals. Microvascular hemorrhage, thrombosis, hypotension, oliguric renal failure, cardiovascular collapse, and coma can occur terminally. FIGURE 30-4 Ocular complications in dogs with RMSF. A, Conjunctival hyperemia and scleral injection. B, Retinal hemorrhages.
Rocky Mountain Spotted Fever
Etiologic Agent
Epidemiology
Incidence and Geographic Distribution
Clinical Features
Physical Examination Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rocky Mountain Spotted Fever
Only gold members can continue reading. Log In or Register to continue
