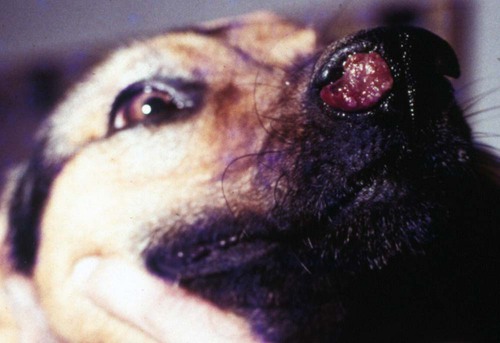
Rhinosporidiosis, a chronic granulomatous disease caused by Rhinosporidium seeberi, induces tumor-like growths of epithelial tissues in domestic animals, birds, and people.9,18,18 The taxonomy of R. seeberi has long been controversial. Based on its morphologic characteristics, it has been classified in the past few years as a fungus by most microbiologists. Phylogenetic analysis indicates R. seeberi to be a member of a newly recognized group of human and animal pathogens that form a branch in the evolutionary tree near the animal-fungal divergence.12,14 It has been proposed that this new phylogenetic group be referred to as the class Mesomycetozoea (between fungi and animals) and R. seeberi be considered a monotypic genus within this class.14,25 Extensive genetic sequence analyses of the 16S rRNA gene indicated 99% similarity with that found in the chloroplasts of flowering plants.2a Genetic analysis of isolates suggests that host-specific strains of the organism may exist.31 Like other mesomycetozoeans, R. seeberi is associated with aquatic environments. Although R. seeberi is generally believed to be the causative agent of rhinosporidiosis, a few challenge this accepted premise. In one study, a cyanobacterium Microcystis aeruginosa was isolated from water in which human patients with the disease were bathing.2,3 The organism was also identified in clinical specimens; however, additional confirmation is needed for this hypothesis. Microscopic findings of lesions indicate that R. seeberi, and not a bacterium, is the cause of this disease. Although a few successful attempts to propagate R. seeberi in tissue culture have been described,9,19 the results have been questioned.24 Most microbiologists consider R. seeberi to be intractable to culture.12,14,14 The infective unit is a small (7- to 15-µm), round spore that, once implanted in tissues, progressively develops into large (100- to 450-µm), spherical bodies known as sporangia. Sporangia undergo a maturation process, resulting in the production of 16,000 to 20,000 endospores that are then discharged through an apical pore, and the cycle is reinitiated.9,23 Although the organisms are not thought to be transmitted directly, they are environmentally resistant; anti-rhinosporidial disinfectants may be indicated in cleaning up following handling or surgery on infected patients. Clinically useful biocides are chlorhexidine, 70% ethanol, povidone-iodine, and silver nitrate for at least 7 minutes contact time.5 Rhinosporidiosis is endemic in India, Sri Lanka, and Argentina and is reported sporadically from other parts of the world. In the United States, canine rhinosporidiosis has been reported mostly in the southern states; however, occurrence of the disease in dogs native to Ontario, Canada, Wisconsin, and Minnesota16,23 suggests the possibility of a more widespread distribution in North America. In Europe, canine rhinosporidiosis has been recognized in northern Italy7 and northwestern England.27 Feline rhinosporidiosis has been reported in three outdoor cats in the mid-Atlantic coastal region of United States.10,28,28 The disease is more common in large-breed dogs with a mean age of 5 years (and a range of 1.5 to 13 years) and seems to be more common in males, as it is in humans and horses. Animals with exposure to aquatic environments have the highest prevalence. Behavioral and biologic factors may be responsible for this apparent predisposition. Because of the few reported cases, it is not known whether the same tendencies are found in cats. One 10-year-old cat had a concurrent nasal adenocarcinoma with the nasal fungal granuloma.10 The pathogenesis of rhinosporidiosis has not been completely characterized because of the difficulties associated with in vitro propagation of the organism. R. seeberi has not been detected in the environment, and its natural host remains unknown. Reports from endemic areas suggest that infection is acquired by mucosal contact with stagnant water. Mucous membrane trauma may be a predisposing factor. In arid countries, most human infections are ocular, and dust is postulated to be a fomite. In vitro studies suggest that endospore release from mature sporangia is stimulated by mucous secretions.24 Once implanted in tissues, endospores elicit a severe, focal pyogranulomatous reaction. Little is known about the immune response to R. seeberi. A mucoidlike layer with immunogenic properties has been recognized beneath the sporangium’s cell wall.15,24 It has been suggested that it may play a role in the immunology of the disease. Clinical findings include wheezing, sneezing, unilateral seropurulent nasal discharge, and epistaxis. Polypoid lesions may be visible in the nares (Fig. 68-1) and may also be visualized by rhinoscopy in the rostral nasal cavity (Fig. 68-2). Single or multiple polyps ranging in size from a few millimeters up to 3 cm are pink, red, or pale gray and covered by numerous pinpoint white-yellowish granules (sporangia). Polyps may be sessile or pedunculated, and the superficial surface is irregular, glistening, and possibly ulcerated. Duration of clinical signs in reported canine cases ranges between 2 weeks and 8 months.
Rhinosporidiosis
Etiology
Epidemiology
Pathogenesis
Clinical Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine