Michele A. Miller, Peter E. Buss Rhinoceroses (rhinos) are among the most primitive of the world’s large mammals, and in prehistoric times were common large herbivores in North America. Five extant species exist in four genera; in Africa, the white (Ceratotherium simum) and black (Diceros bicornis) rhinos and in Asia, the Sumatran (Dicerorhinus sumatrensis), Indian (Rhinoceros unicornis), and Javan (Rhinoceros sondaicus) rhinos. The Sumatran rhino is the most primitive and predates the extinct woolly rhino (Coelodonta antiqitatis), which inhabited in northern Europe and Asia during the last Ice Age. African rhinos live in habitats that are related to dietary requirements; the white rhino requires relatively flat terrain with areas of short grass, whereas the black rhino prefers areas with shrubs and young trees. The Javan rhino inhabits lowland coastal forests rather than the more mountainous inland areas. Sumatran rhinos are found in areas of dense primary rain forest. Indian rhinos exist across a wide range of habitats, including marshes, alluvial plains, grasslands, and aril forests. All species of rhino require regular access to water. They need to drink daily or every second day, as they are hindgut fermenters with relatively fast gut transit times which reduce the time for water resorption from the feces.35 Rhinos wallow in mud pools to cool off during the heat of the day and to help keep their skin free of external parasites. Habitat loss and severe poaching has led to the devastation of rhino populations. Current worldwide population estimates (2012) are 35 to 44 Javan, 152 to 199 Sumatran, 3270 Indian, 4837 black, and 20,143 white rhinos (International Rhino Foundation; www.rhinos.org). In contrast, in the 1970s, the black rhino population in Africa was approximately 65,000.22 Rhinos have a barrel-shaped torso; short, thick legs and broad feet with three weight-bearing digits; and an elongated, bulky skull. The soles of the feet have a large pad to cushion the weight of the animal. The most distinctive feature of rhino is the presence of a single horn (Rhinoceros sp.) or a pair of horns (Ceratotherium, Diceros, and Dicerorhinus) that are composed of tubular hairlike keratin filaments, which are outgrowths of the skin. The horn is relatively easy to displace, since it is not attached to the skull but rather set on bony protuberances.35 African rhinos lack both incisors and canine teeth: incisors (I) 0, canines (C) 0, premolars (P) 3–4, molars (M) 3); in contrast, the Asian species have incisors: I 1/1–2, C 0, P 3–4, M 3).28 Hypertrophied and tusklike lateral lower incisors are characteristic of the three Asian rhinos, with only members of the genus Rhinoceros having a pair of small central incisors. The black, Sumatran, and Javan rhinos are browsers with prehensile upper lips, which assist in grasping the plants that they consume. White and Indian rhinos have wide, flat lips for grazing. The rhino’s skin is thick, and in the white rhino, it may reach a thickness of 5 centimeters (cm), with a thick vascular dermis covered by an epidermis 1 millimeter (mm) thick.35 All rhinos have skinfolds, although these are more pronounced in the Asian species, with the Indian rhino being best known for the exaggerated armorlike plates. The Sumatran rhino is unique, having a distinctive shaggy coat of hair with particularly hairy ears and a tuft of long hair at the tip of the tail. Enclosures for all rhino species need to be sturdy and constructed of concrete, large-diameter wooden or metal poles or of more natural materials such as rocks; however, it should ensure that the animals cannot climb over or become entrapped within the enclosures. Spacing between vertical bars should be 0.5 m. If calves are present, chains or cables should be added. When horizontal poles are used, a potential for horn avulsion or climbing exists. Substrate should be textured to minimize slipping yet not abrade foot pads. Most indoor stalls are made of concrete to facilitate cleaning. Natural substrate used in outdoor enclosures or pens should allow adequate drainage and cleaning or be periodically changed to prevent buildup of parasites, pathogens, or excessive moisture. All rhinos must be provided with access to pools and wallows. The access and depth of the pool should be sufficient to encourage use and allow complete submersion, especially for Indian and Sumatran rhinos. Enclosure items such as scratching posts, rocks, and vegetation promote natural, species-specific behaviors but should be designed to avoid head or limb entrapment or horn rubbing or avulsion. Rhinos may be acclimated to cold and inclement weather if provided access to shelter during wet and windy conditions. However, supplemental heat (to maintain 13° C) should be provided in cold climates when temperatures consistently drop below 10° C. Ill, old, or young animals may need higher temperatures. Of the four rhino species, white rhinos are the most social and usually housed in groups. Black rhinos are more solitary but have been housed in small family groups in captivity. Indian rhinos are mostly solitary. Therefore they should be kept as individual animals except in very large spaces. Sumatran rhinos should also be housed individually and only introduced for breeding purposes. Significant aggression during breeding may occur with black, Indian, and Sumatran rhinos, and adequate space should exist to prevent cornering of animals. Feeding strategies differ by species. Black and Sumatran rhinos are browsers, white rhinos are considered grazers, and Indian rhinos are classified as intermediate feeders. Studies on digestibility in captive rhinos show that the horse is a useful model for the development of diets for Indian and white rhinos but not for black rhinos.7 Captive black rhinos appear to receive higher proportions of concentrates compared with other species and would benefit from higher proportion of browse. In captivity, a rough guideline for diet quantity is 1% to 3% body weight as fed, with no more than one third of total calories obtained from pellets.22 Energy management for weight control is especially important in Indian rhinos; 0.5% to 1.1% body weight in dry matter (DM) is adequate in this species.9 Grass hay should be fed to white and Indian rhinos, whereas a grass–legume mixture or a legume–browse mixture is used for black and Sumatran rhinos. Alfalfa fed as exclusive forage may lead to mineral imbalances, colic, and diarrhea. Black rhinos appear to have higher calcium (Ca) and magnesium (Mg) absorption compared with horses and higher fecal losses of sodium (Na) and potassium (K).7 Therefore, excessive mineral supplementation should be avoided. Natural browses appear to be limited in Na, phosphorus (P), zinc (Zn), and selenium (Se). Appropriate Ca : P ratio should also be monitored, since low phosphorus values have been associated with poorly defined syndromes, including dermatologic and hematologic disorders, in black rhinos.10 Vitamin E deficiency has been linked to health issues, especially in black rhinos. Therefore, the contents of the diet should be analyzed to ensure a sufficient concentration (150–200 international units per kilogram [IU/kg] DM).22 Black and Sumatran rhinos are susceptible to iron storage disorder in captivity, so a high-fiber, low-iron diet should be provided to these species. A low-iron herbivore pellet, in addition to browse, fed to captive black rhinos resulted in a decrease in serum ferritin levels.27 Low-iron diets consisting of browse, long-stem forage, and low-iron pellets (iron [Fe] ≤350 parts per million [ppm]) is recommended for browser rhino species (Valdes E, personal communication). Citrus and other produce containing vitamin C may enhance iron absorption and should be minimized in the diets of these species. Analyses of rhino milk have shown that it is lower in total solids compared with the milk of most ungulates (8.2%–8.8%). The relative composition is high in sugars (63%–82% of total solids), with 14%–28% protein and low fat (2.6%–6.8% of total solids).3 Formulas used for hand-raising rhinos are based on cows’ milk or commercial formulas (ZooLogic Milk Matrix 20/14, PetAg, Inc., Hampshire, IL). Lactase (Lactaid) has been useful as a milk additive in these cases. Indian, white, and black rhino calves have been successfully hand-reared with these formulas. In neonates that have not received colostrum, bovine or equine colostrum should be fed at a 50% dilution to provide immunoglobulins if rhino colostrum is unavailable. Addition of 10% colostrum to the formula for up to 1 month is recommended for local gastrointestinal (GI) immunity.3 Feeding for the first 3 days is 10% of body weight (kg) divided into seven feedings, with an increase to 15%–20% on day 4 through 6 months of age. At 6 months through the start of weaning at 1 year, a constant volume of 11 kg of formula at each of three feedings should be offered. Calves should be weighed regularly and gain 1 to 2 kg/day. Chutes for rhinos may be simple free-stall designs, in which an individual animal is trained to enter voluntarily and poles or bollards allow protected contact. More sophisticated designs include hydraulic or movable walls, head restraints, and access doors for examinations and minor medical or reproduction-related procedures. Ideally, the design should be incorporated into the facility so that the animal has to pass through and it is not a dead-end. Successful use of these devices usually requires operant conditioning of the rhino, the use of tranquilization or sedation, or a combination. This section will focus on restraint of captive rhinos. Excellent sources of information on the immobilization of free-ranging rhinos are available.5,35 The two techniques used are standing restraint and recumbent immobilization. For captive rhinos, drug delivery usually requires use of darting equipment. Depending on the situation, pole syringes with robust needles or hand-injection in conditioned animals may be used. Most darting systems may be used in captive situations, as long as a robust needle (minimum 40–60 mm × 2 mm needle) is used to penetrate the thick skin and deliver the drug intramuscularly. Nylon darts (Teleinject, Daninject) are preferred in these situations, since they cause less trauma compared with metal darts. Ideal sites for drug injection are just caudal to the ear on the lateral cervical area, upper caudal hindlimb, shoulder, or nuchal hump in the white rhino. However, any site may be used if the dart is placed perpendicular to the skin and is adequate to penetrate muscle. Depending on the animal’s health status, the environment, and the procedure planned, food and water should be withheld prior to the procedure, at least overnight, although regurgitation and aspiration are infrequent complications in rhinos. Procedures should be planned for the coolest time of day, preferably early mornings. Rapid induction and minimal excitement further decrease the risk of hyperthermia. Rectal enemas, evaporative cooling with sprayers and fans, or cold water baths for small individuals are warranted if the rectal temperature is greater than 39° C, although complete anesthetic reversal should be performed immediately if the temperature reaches 41° C or above.5 Rhinos are prone to developing myopathy and neuropathy after prolonged recumbency. Inflated truck inner tubes, heavy mats, or padding may be used under pressure points to prevent radial nerve paralysis and other neuropathies if the procedure is to take place on a hard surface. The optimal body position is still being debated. Lateral recumbency is often preferred, since it provides optimal circulation to the limbs; however, sternal recumbency may allow better ventilation. If the animal needs to be in sternal recumbency for the procedure, it is ideal to roll the animal into lateral recumbency, whenever possible, to ensure adequate peripheral circulation. Limbs should be “pumped” about every 20 minutes to promote perfusion of muscles.35 It is imperative that immobilized rhinos be closely monitored to minimize complications. Hypoxia, hypercapnia, and hypertension are commonly associated with the use of potent opioids in rhinos.22,33-Accurate weight or estimated weight facilitates optimal drug calculation and prevents drug overdosing or underdosing and associated problems. Pulse oximetry is useful for monitoring trends in hemoglobin oxygen saturation. Sites for placement include the ear pinnae (scraping of the skin surface may sometimes provide more accurate readings), mucosal folds of the prepuce, vulva, and rectum; and sensor pads placed side by side in the conjunctival sac, gingival mucosa, nasal mucosa, and inside the rectum, vagina, or prepuce. Ideally, readings should be greater than 90%, but interpretation must be made in conjunction with the color of the mucous membranes and blood and other clinical signs. Capnography may be used by placing a small-animal endotracheal tube inside a nostril in nonintubated rhinos. Direct and indirect blood pressure may be measured by using either an arterial catheter in the medial auricular artery or a blood pressure cuff at the base of the tail, respectively. Standing sedation should only be attempted after proper consideration of animal and staff safety. In captive rhinos, standing chemical restraint has historically been performed using low doses of the potent opioid etorphine. However, variable responses may lead to recumbency. More recently, butorphanol, alone or in combination with azaperone or α2-agonists (detomidine, medetomidine), has been successfully used. In the author’s experience, a combination of etorphine (1 mg /1000 kg), butorphanol (10 mg butorphanol to 1 mg etorphine), and azaperone (20 mg standard dose, intramuscularly [IM]) has been effective in “walking” captive white rhino, with the use of a white flag as a target to bring the rhino from the holding facility to the crate. Once in the crate, the animal will remain standing and tolerate minor procedures. Chemical restraint may be partially to fully reversed with naltrexone (40–100 mg naltrexone:1 mg etorphine or 1–2.5 mg naltrexone:1 mg butorphanol) with or without atipamezole (5 mg atipamezole:1 mg α-2-agonist), depending on the drug combination chosen (Box 55-1).22,33,35 Potent opioids are the primary drugs used for general anesthesia in rhinos. Etorphine is mostly commonly used, although carfentanil (in some species, this has been suggested to cause excitable inductions) and, more recently, combinations of etorphine and thiafentanil have also been administered to rhinos. Opioids are typically combined with azaperone, α2-agonists, or acepromazine to decrease complications. Hyaluronidase may be included to increase absorption and decrease induction time. To deepen the anesthesia or for prolonged procedures, supplemental doses of etorphine, ketamine, propofol, or inhalant anesthetics have been used. Midazolam, diazepam, and guaifenisen infusion may provide additional muscle relaxation. Suggested doses for recumbent immobilization in captive rhinos are given in Box 55-2.5,22,33–35,41 White rhinos and, to a lesser extent, Indian rhinos appear to be more sensitive to the effects of opioids compared with black rhinos and exhibit muscle tremors, limb paddling, hypoxia, hypercapnia, and hypertension.33,35 Butorphanol has been administered to antagonize respiratory depressive effects in white rhino (10–20 : 1 mg butorphanol to etorphine ratio); however, it may also lighten the plane of anesthesia.21 It should be used with caution in black rhinos, since they appear to be more sensitive and may suddenly get to their feet.35 Other partial opioid agonist–antagonists are routinely used in the field and may be adapted for captive rhinos, when available (e.g., nalbuphine). Butorphanol–azaperone and butorphanol–medetomidine or detomidine combinations have successfully induced recumbency in captive Sumatran, Indian, and white rhinos.34,35,41 Oxygen supplementation by intratracheal intubation or nasal insufflation (flow rates of 15–30 liters per minute [L/min]) may increase oxygen saturation values.24 Doxapram has been administered for apnea in rhinos but may only provide short-term relief. Partial or complete reversal should be considered in severe cases of hypoxia. On some occasions other than medical procedures, rhinos may need to be sedated, as for crating and transport. For short-duration tranquilization or sedation, benzodiazepines (2–6 hours; adult doses: midazolam 5–50 mg, IM; diazepam 10–30 mg, IM) and azaperone (2–4 hours; 80–200 mg, IM) are useful choices in rhinos. Long-acting neuroleptics (LANs) are typically administered in free-ranging rhinos after capture for transport and boma acclimation, although they have also been used in captive rhinos for longer-duration tranquilization. Zuclopenthixol acetate (Clopixol-Acuphase) at doses of 60 to 200 mg lasts 3 days, and perphenazine enanthate (Trilafon-LA) does not take effect for 12 to 18 hours but lasts 7 to 10 days (100–200 mg, IM).5 Most surgical procedures involve the skin, eyes, digits, and horn, including treatments for corneal ulcers, cataracts, pododermatitis, wounds, and tumors. Because of the thickness and inelasticity of the rhino skin, suturing of wounds often results in dehiscence, so unless necessary, wounds are often left to heal by secondary intention. Use of wire sutures, stents, and mattress patterns may improve closure of the rhino skin. Surgical management of horn and integument problems, including tumors, may be achieved with the use of operant conditioning, standing sedation, or full immobilization. Surgical treatment of ocular problems, including corneal ulcers and cataracts, is fairly common. Management of pododermatitis by surgical debridement has also become a more routine procedure, especially in Indian rhinos. Treatment of osteomyelitis in a black rhino, involving surgical debridement and vacuum-assisted closure, has been recently described.16 Surgical repairs of rectal prolapse in black and Indian rhinos and patent urachus in a white rhino calf have been reported. Although most abdominal surgeries are unsuccessful, an adult white rhino has survived an exploratory celiotomy.40 Laparoscopic techniques for rhinos are still in their developmental stages but have been successfully used for reproductive procedures such as uterine biopsy and oocyte retrieval.18 With the exception of studies on anesthetics and a few vaccines, no pharmacokinetic trials in rhinos have been performed. Most clinicians use the horse as the model to determine drug dosages, especially for antibiotics and antiparasitics. Commonly used antibiotics include oral trimethoprim–sulfadiazine equine formulations, parenteral large-animal cephalosporins, and oral fluoroquinolones. Antiparasitics are not routinely required in captive rhinos, but oral and injectable ivermectin; oral fenbendazole, pyrantel pamoate, and niclosamide; and pour-on acaricides, including flumethrin 0.5%, have been used in rhinos.22 Nonsteroidal anti-inflammatories are frequently prescribed for analgesia. Rhinos should be trained to permit sample collection and clinical examination. Resting values for heart rate, respiratory rate, temperature, and other values have been obtained for nonrestrained black and white rhinos. The various species appear to be similar with regard to heart rates (30–40 beats per minute) and respiratory rates (6–12 breaths per minute). Rectal temperatures are typically 34.5° C to 37.5° C, although temperatures may be higher in anesthetized rhinos (37° C–39° C) because of exertion or muscle tremors.22,35 Limited information on electrocardiography (ECG) values in rhinos is available.19 Indirect blood pressure has been measured in unsedated black and white rhinos by using a human blood pressure cuff around the base of the tail. Mean values reported for unanesthetized white rhinos are 160 +/−2.9 millimeters of mercury (mm Hg; systolic), 104 +/−2.3 mm Hg (diastolic), and 124 +/−2.2 mm Hg (mean blood pressure).6 In anesthetized animals, etorphine may cause hypertension, but some authors have observed more variable mean blood pressure values (107–280 mm Hg) depending on the drugs used and when measurements were taken. Hematologic and biochemical parameters have been published (Tables 55-1 through 55-3).14,22 Although most values may be interpreted as being similar to other perissodactyls, total protein and globulins tend to be higher and sodium and chloride lower than in domestic horses. Hypophosphatemia (low blood phosphorus) is a recognized problem in captive black rhinos, with levels dropping below 1 milligram per deciliter (mg/dL). Low serum phosphorus has been linked to hemolytic anemia and other blood disorder syndromes.10,22 Supplemental doses of elemental phosphorus (preferably chelated) (10 to 24 g, orally [PO], once daily [SID]) are used in black rhinos until normal serum levels are reestablished, as reported anecdotally (Valdes E, personal communication). In critical cases, intravenous sodium or potassium phosphate may be administered at 14.5 millimoles per hour (mmol/hr), but serum calcium should be carefully monitored. TABLE 55-1 Mean Hematology Values in Rhinos (±SD)14
Rhinoceridae (Rhinoceroses)
Biology
Unique Anatomy
Special Housing Requirements
Feeding and Nutrition
Restraint and Handling
Physical Restraint
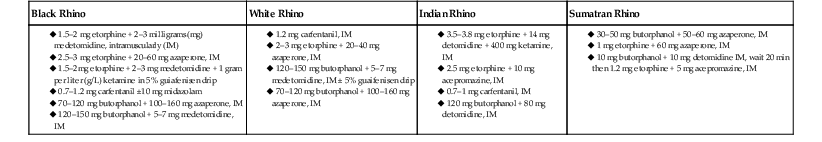
Chemical Restraint
Surgery
Other Pharmaceuticals
Physical Examination and Diagnostics
Parameter
Black Rhino
White Rhino
Indian Rhino
Sumatran Rhino
White blood cell (WBC) × 103/microliter (µL)
8.42 (2.48)
9.30 (2.46)
7.20 (1.33)
8.27 (1.55)
Red blood cell (RBC) ×106/µL
4.01 (0.88)
5.77 (1.28)
6.43 (0.86)
5.32 (1.09)
Hemoglobin, gram per deciliter (g/dL)
12.0 (2.0)
13.8 (3.8)
13.4 (1.5)
12.4 (1.6)
Hematocrit %
33.4 (5.7)
36.9 (9.3)
37.0 (4.6)
36.9 (4.2)
Mean corpuscular volume, (fL)
85.7 (9.0)
63.8 (7.8)
57.8 (4.9)
71.5 (11.2)
Mean corpuscular hemoglobin, picogram per cell (pg/cell)
30.5 (3.3)
23.5 (1.9)
21.3 (3.0)
23.9 (3.8)
Mean corpuscular hemoglobin concentration (g/dL)
35.7 (2.7)
37.9 (7.3)
36.3 (3.2)
33.5 (2.1)
Platelets ×103/µL
284 (83)
378 (103)
178 (53)
198 (135)
Nucleated RBC/100 WBC
0
1 (1)
0
—
Reticulocytes %
1.6 (2.9)
—
—
—
Neutrophils ×103/milliliter (mL)
5.24 (2.18)
5.42 (2.05)
5.13 (1.24)
4.86 (1.16)
Lymphocytes ×103/mL
2.48 (1.1)
2.35 (1.15)
1.74 (0.67)
2.52 (0.90)
Monocytes ×103/mL
0.43 (0.32)
0.65 (0.55)
0.22 (0.15)
0.36 (0.22)
Eosinophils ×103/mL
0.25 (0.22)
0.54 (0.59)
0.32 (0.31)
0.37 (0.21)
Basophils ×103/mL
0.17 (0.09)
0.10 (0.05)
0.13 (0.05)
0.08 (0.01)
Neutrophilic bands ×103/mL
0.27 (0.35)
0.71 (1.18)
0.22 (0.20)
0.31 (0.24) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rhinoceridae (Rhinoceroses)
Chapter 55