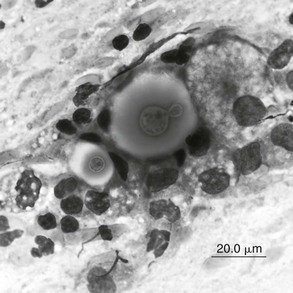
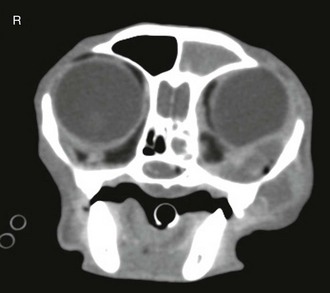
Chapter 155 Sneezing and nasal discharge in the cat can result from infectious, inflammatory, or neoplastic disease. The most common syndrome is likely chronic rhinosinusitis (CRS), a disease with high morbidity in the feline population but uncertain etiopathogenesis. Multiple clinical reports have implicated a role for feline herpesvirus type 1 (FHV-1) in CRS; however, the high prevalence of infection with this virus and the ability of the virus to cause latent infection in the feline population have made it difficult to demonstrate a causal relationship between the presence of the virus and disease (Johnson et al, 2005). In addition, cats with CRS often demonstrate improvement in response to antibiotics, which suggests that bacterial infection plays a role in this disease. It is possible that severe early viral infection, viral persistence within nasal epithelium, or chronic reactivation of FHV-1 in nasal tissues damages the respiratory epithelium and results in mucus accumulation, sneezing, and ultimately turbinate destruction with subsequent bacterial invasion of nasal tissue. Fungal infection most commonly is caused by cryptococcal species (Cryptococcus neoformans–Cryptococcus gattii species complex), although more recently, Aspergillus felis in the Aspergillus fumigatus complex has been recognized as causing nasal and orbital infections in cats (Barrs et al, 2012). Secondary bacterial infection due to tooth root disease or an oronasal fistula can be a cause of nasal discharge, and foreign body impaction should be considered as a cause of unilateral nasal disease, although this is much less common in cats than in dogs. Finally, nasal discharge can result from systemic or pulmonary disease, particularly with nasal regurgitation of material associated with either chronic vomiting or pneumonia, or with epistaxis due to systemic hypertension, hyperviscosity, or a coagulopathy. For cats with unilateral nasal discharge and loss of nasal airflow, fungal infection and neoplasia should be high on the differential list. Cytologic analysis of nasal discharge is occasionally diagnostic for cryptococcosis by detection of a large (~20- to 30-µm) clear capsule surrounding a 3- to 8-µm organism (Figure 155-1). Cryptococcal antigen latex agglutination serologic testing should also be performed in cats with a loss of nasal airflow, since detection of circulating cryptococcal polysaccharide capsular antigen indicates infection. The assay has a high sensitivity (96% to 98%) and specificity (97% to 100%) when a pronase step is included in the method (pronase degrades antibodies that bind to the capsular antigen) (Malik et al, 1996; Trivedi et al, 2011). Fungal culture of a nasal swab also is indicated to isolate organisms for subsequent identification of species, antifungal susceptibility, and molecular subtype at a mycology reference laboratory. Nasal culture should not be used as a single diagnostic test for cryptococcosis, since it is possible for the nasal cavities of cats to be transiently colonized by cryptococcal organisms and not infected (Duncan et al, 2005). Figure 155-1 Smear from a nasopharyngeal cryptococcal granuloma treated with modified Wright-Giemsa (Diff-Quik) stain. The organisms, including a single budding yeast, are surrounded by a large polysaccharide capsule. Measurement of serum levels of galactomannan, a polysaccharide component of fungal cell walls, is a noninvasive test used in the early diagnosis of human invasive aspergillosis (IA). It was evaluated recently for detecting upper respiratory tract (URT) aspergillosis in cats and was found to perform poorly, with a sensitivity of 23% and specificity of 78%. Immune-mediated clearance of galactomannan antigen is suspected to be a major reason for the poor sensitivity of this test in affected cats (Whitney et al, 2012). Antibodies against Aspergillus species were detected in the sera of four of nine cats with URT aspergillosis in several case reports, but the diagnostic usefulness of this serologic test has not been fully evaluated. After laboratory testing (including tests for feline leukemia virus and feline immunodeficiency virus in cats with unknown viral or vaccination status), the first step in the diagnostic workup is to anesthetize the cat for skull radiography or preferably computed tomography. These images show variable degrees of turbinate lysis and increased fluid density within the nasal cavity in either rhinitis or neoplasia. The middle ear and sinuses are often fluid filled because of blocked drainage. Neoplasia and fungal infections usually have unilateral findings of a mass effect or soft tissue density. With invasive sino-orbital aspergillosis, an irregularly contrast-enhancing retrobulbar mass, paranasal soft tissue mass effect, and bony lysis generally are seen (Figure 155-2). Figure 155-2 Transaxial contrast-enhanced computed tomographic scan for a cat with sino-orbital aspergillosis. There is a mass in the ventromedial orbit causing dorsolateral displacement of the globe as well as opacification of the left frontal sinus, left sphenoid sinus, and choanae. The mycotic granuloma in the orbit has spread to the paranasal soft tissues adjacent the maxilla. The pattern of contrast enhancement is heterogenous, with encapsulated areas of peripheral rim enhancement. Rhinoscopy with biopsy and dental probing are performed after imaging to avoid creating artifacts on radiographs or CT. First, nasopharyngoscopy is accomplished by retroflexion of a flexible endoscope above the soft palate to rule out nasopharyngeal stenosis, foreign body, or a mass lesion. Neoplasia and fungal infections are often found in the choana and can be diagnosed with visualized sampling of the region for histopathology. Rhinoscopy of the rostral nasal cavity is best performed using a small (<2.8-mm) rigid telescope. Mucoid or purulent discharge is common in all causes of nasal discharge. Cats with CRS tend to have destructive rhinitis with loss of turbinates; in contrast, fungal infection or neoplasia typically results in a mass lesion. Cats with aspergillosis can display a mass effect or occasionally have cavitated rhinitis and fungal plaques similar to those in dogs with the condition. Plaques or biopsy specimens from mass lesions should be submitted for fungal culture when infection is suspected. In patients with frontal sinus involvement and sinuses of adequate depth as assessed by CT, sinus trephination using a Jacob chuck and intramedullary pin (3.2 to 4 mm in diameter) enables endoscopic examination of the lateral compartment of the frontal sinuses and ready access to material for culture, histologic examination, and polymerase chain reaction (PCR) testing. Standard laboratory media such as Sabaraud’s dextrose agar or cornmeal agar are adequate for isolation of most fungal pathogens of the upper respiratory tract. Differentiation of A. fumigatus from other species in the A. fumigatus complex often is not possible based on morphologic features alone but can be achieved using molecular techniques. A panfungal PCR procedure that amplifies the internal transcribed spacer 1 (ITS1) region of the ribosomal DNA gene cluster between the 18S and 5.8S ribosomal RNA genes can be performed using DNA extracted from formalin-fixed or fresh tissue biopsy specimens. Alternatively, using DNA extracted from fungal culture material, a panfungal PCR procedure that targets a larger region of the ribosomal DNA gene cluster including the ITS1, 5.8S gene, and ITS2 regions can be used and is more likely to yield reliable identification. These regions of the fungal genome are useful to amplify because they are multicopy (≥100 copies in the fungal genome) and they contain highly variable regions that facilitate identification of a diverse range of fungal genera from clinical specimens, including both filamentous fungi and yeasts. Comparative gene sequence analysis of an additional gene region such as the partial beta-tubulin or calmodulin genes may be necessary for definitive identification of some Aspergillus species. PCR products are sequenced and then compared with those in the National Institutes of Health GenBank database to obtain the identity of the fungus (Barrs et al, 2012). Collecting nasal swab or biopsy specimens for bacterial culture in cats in which CRS is suspected is controversial. Potential pathogens are isolated from nasal samples from cats with chronic rhinitis more commonly than from those from healthy cats (Johnson et al, 2005), and culture results are sometimes helpful in guiding antibiotic therapy to control secondary bacterial rhinitis. It is unclear whether it is of clinical value to determine the type of inflammation present in CRS; however, histopathologic analysis is critical for ruling out neoplasia or fungal infection. In the cat with CRS, lymphoplasmacytic inflammation likely reflects the chronic nature of disease, whereas neutrophilic inflammation indicates an acute or bacterial component to the disease process. Eosinophilic infiltrates are relatively uncommon, but this finding might be considered suggestive of herpesvirus infection, as has been found in FHV-1–related facial dermatitis (Hargis et al, 1999). Since eosinophilic infiltrates can also occur in fungal rhinitis, special stains to detect fungal elements (e.g., periodic acid–Schiff or Gomori’s methenamine silver) should be used.
Rhinitis in Cats
Diagnosis
Rhinitis in Cats
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


