Chapter 4 Respiratory Diseases
DISEASES OF THE UPPER AIRWAY
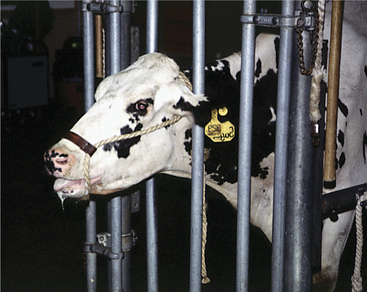
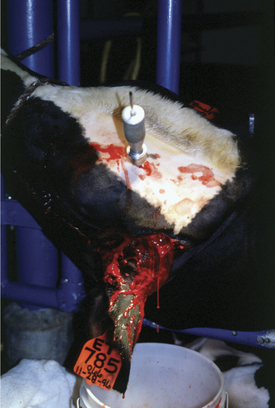
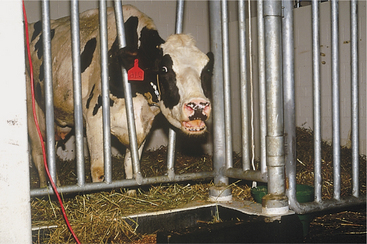
These disorders are characterized by inspiratory dyspnea. The increased resistance to airflow caused by upper airway obstructions often creates audible inspiratory noise and results in referred airway sounds through the tracheobronchial apparatus. Sounds that have been “referred” to the lower airway from an upper airway obstruction may be misinterpreted as lower airway in origin unless the upper airway is examined and the trachea ausculted in such cases. If the respiratory sounds can be heard without a stethoscope, they are most likely originating from the upper respiratory tract. The upper airway examination should include detection of airflow from both nostrils, close examination of soft tissues of the head, and oral examination if necessary. Severe upper airway obstruction can cause open mouth breathing and head extension as the affected cow tries to decrease the resistance to airflow (Figure 4-1).
Mechanical or Obstructive Diseases
Congenital
Diagnosis.
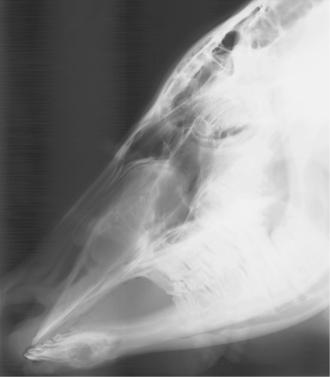
Specific diagnosis requires physical examination, including visual inspection of the nares and oral cavity, endoscopy, and skull radiographs (Figure 4-2). In addition, aspiration for cytology and cultures may be indicated for cystic lesions. Most cystic lesions will be secondarily infected.
Treatment.
Regardless of cause, symptomatic or supportive treatment may be necessary before diagnostic procedures are performed in calves with severe dyspnea, lest the stress of examination or endoscopy induce anoxia. A tracheostomy should be considered to allow safe diagnostic manipulation. Misinterpreting anoxic patient struggling as wildness requiring additional physical restraint is a frequent, and potentially fatal, error in judgment made by inexperienced clinicians. When a dyspneic animal struggles during examination, usually it is anoxic, frightened, and extremely anxious. All restraint of the head and neck should be relaxed, and the animal should be allowed to “get its breath.” Continued restraint during these situations will result in asphyxiation of the animal.
Acquired
Etiology and Signs.
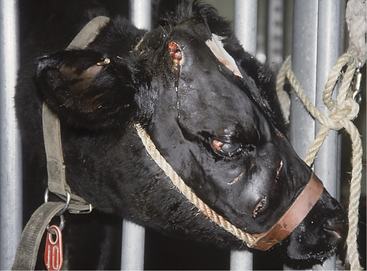
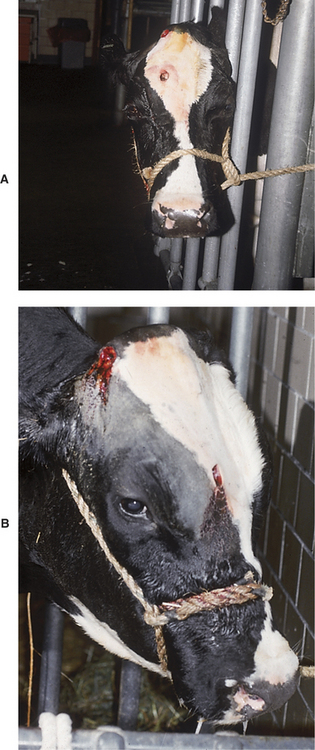
Regardless of cause, progressive inspiratory dyspnea is the primary sign observed in affected cattle. Fever may be present with pharyngeal abscesses or chronic maxillary sinusitis. Unilateral nasal discharge or reduced airflow from one nostril may be present with maxillary sinusitis or unilateral neoplasms of the nasal pharynx or maxillary sinus. Lymphadenopathy may be present as a primary sign in neoplastic conditions, such as juvenile lymphosarcoma and adult lymphosarcoma (Figures 4-3 and 4-4), or as a secondary sign, in cases of soft tissue infections. Unilateral Horner’s syndrome and progressive exophthalmos have been observed in slow-growing adenocarcinomas of respiratory epithelial origin in the nasal pharynx (Figure 4-5). Cattle with unilateral nasal obstruction often show more obvious respiratory signs during hot weather. One cow with Horner’s syndrome would demonstrate open mouth breathing on hot days because of the nasal mucosal vasodilation and edema (Figure 4-6). A fetid odor may exist on the breath caused by chronic inflammation or tumor necrosis in some cattle. The owner may report a progressive course of stertorous breathing eventually leading to open mouth breathing. Inflammatory lesions often have a more acute course than neoplasms, but this is a generality rather than a rule. Obvious external swelling may be present in certain conditions such as chronic maxillary sinusitis, pharyngeal or retropharyngeal abscesses, and lymphosarcoma.

Figure 4-4 Adult Holstein with lymphosarcoma mass in the pharyngeal area that caused inspiratory dyspnea.
Treatment and Prognosis.
In general, neoplasms have a hopeless prognosis, and the animal should not be treated. Juvenile lymphosarcoma often causes upper airway dyspnea via enlarged pharyngeal lymph nodes. Occasional adult-form lymphosarcoma cases have one or more very large (10 to 20 cm diameter) pharyngeal or mediastinal lymph nodes that will cause dyspnea. Lymphosarcoma usually results in death within 1 to 6 months of diagnosis. Adenocarcinomas originating in the respiratory pharynx in older cattle (i.e., more than 8 years of age) may have an insidious but progressive course over months to years. Therefore unlike cattle with lymphosarcoma, these animals may be allowed to survive for some time to deliver another calf or to undergo superovulation and embryo transfer. Only if the animal stops eating, develops severe respiratory distress, or is suffering from exposure damage from an exophthalmic eye will euthanasia be necessary. Cattle affected with primary squamous cell carcinoma, metastatic squamous cell carcinoma, or osteosarcoma originating in a sinus, bone, or periocular location occasionally may have enough tumor mass or lymph node metastases to develop inspiratory dyspnea. Cattle with squamous cell carcinomas frequently have a fetid breath odor from the primary tumor and should not be made to suffer unduly.
Inflammatory Diseases
Granulomas Caused by Actinobacillus lignieresii or Actinomyces bovis
Etiology and Signs.
Actinobacillus lignieresii granulomas within the nasal cavity usually are unilateral masses within the external nares and appear as red, raised, fleshy masses that bleed easily and look very similar to Rhinosporidium granulomas (Figure 4-7, A and B). Signs include a progressively enlarging mass in one nostril and inspiratory dyspnea as the lesion enlarges to occlude the nostril completely. The granulomas may originate at the site of nose-lead lesions of the mucosa near the nasal septum or at other mucosal sites of soft tissue injury from foreign bodies or fibrous feed. Progressive inspiratory dyspnea and nasal discharge are found in patients having granulomas deeper in the nasal cavity, larynx, pharynx, or trachea. Actinomyces bovis was responsible for multiple tracheal granulomas in a cow treated at our clinic.
Treatment.
Treatment for granulomas caused by A. lignieresii consists of excisional biopsy to debulk the mass to the level of nasal mucosa and sodium iodide therapy until iodism is observed. Usually this requires IV sodium iodide (30 g/450 kg) initially and at 2- to 3-day intervals for several treatments, or oral organic iodide (30 g/450 kg) daily following the initial IV dose. Cryosurgery has been used successfully on these granulomas following debulking. In severe or recurrent cases, antibiotic therapy may be necessary in addition to sodium iodide. Penicillin and ampicillin have been used to treat infection caused by A. lignieresii. Whenever possible, an antibiotic should be selected based on organism culture and sensitivity results. Usually the prognosis is good.
Frontal Sinusitis
Etiology and Signs.
Chronic frontal sinusitis does not develop until months to years following dehorning and may be completely unassociated with dehorning because it occasionally occurs in animals dehorned by noninvasive techniques, polled animals, or animals with horns. Ascending respiratory tract infections, as in other species, are a cause of chronic frontal sinusitis and usually are caused by P. multocida. Chronic frontal sinusitis associated with old dehorning complications such as low-grade infection, bony skull fragments, or sequestra typically is associated with infection by A. pyogenes or mixed infections that may include A. pyogenes, P. multocida, anaerobes, or miscellaneous gram-negative organisms. Signs of chronic frontal sinusitis include gradual loss of condition and production that may be constant or intermittent; unilateral nasal discharge usually is observed, again as a persistent or intermittent complaint. Additional signs include head pressing, an extended head and neck, partially closed eyes, or resting of the muzzle on inanimate objects, all of which signal headache or pain. Intermittent or consistent fever is present. Bony expansions of the sinus may occur, causing asymmetric facial distortion—especially in cattle that do not have significant nasal discharge because of occlusion or obstruction of the ethmoidal meatus opening into the nasal cavity. In fact, some cattle will have intermittent bony swelling of the sinus that becomes less apparent during times of sinus drainage with subsequent nasal discharge. Palpation or percussion of the frontal bone overlying the affected sinus causes pain, and the patient is extremely apprehensive when the examiner approaches the head. Bony expansion of the sinus may result in ipsilateral exophthalmos and decreased air movement through the ipsilateral nasal passage (Figure 4-8). Neurologic complications, including septic meningitis, dural abscesses, and pituitary abscesses, are possible in neglected cases as a result of erosion of the bony sinus. Tetanus is another potential complication. Occasionally cattle with chronic frontal sinusitis have developed orbital cellulitis, pathologic exophthalmos, or facial abscesses from infectious destruction of the postorbital diverticula of the sinus, allowing soft tissue infection of the orbit (Figure 4-9).
Diagnosis.
Diagnosis of chronic cases may be possible based only on clinical signs coupled with palpation and percussion of the sinus in selected cases. When mature animals are affected, however, it is important to rule out neoplasia and other differentials. Skull radiographs are helpful when available. Drilling into the sinus with a Steinmann’s pin and collection of purulent material for cytology and bacterial cultures will confirm the diagnosis (Figure 4-10). Sedation and local anesthesia allow this procedure to be performed with minimal patient discomfort.
Treatment.
Treatment of chronic frontal sinusitis requires trephination of the sinus at two sites to allow lavage and drainage. One site is at the cornual portion of the sinus, and the second is located over the affected sinus approximately 4.0 cm from midline and on a transverse line connecting the caudal bony orbits (Figure 4-11, A and B). A third site caudodorsal to the rim of orbit and medial to the temporal ridge has been recommended, but we have found this site to be dangerous because it occasionally results in orbital soft tissue infection as compromised softened bone is penetrated. Further caution regarding trephination of the sinus should be practiced in animals less than 2 years of age because the rostral and medial rostral portions of the sinus may not be developed in younger animals. Attempts to establish rostral-medial drainage in these animals may risk invasion of the calvarium. Drains may be placed to communicate the two trephine sites and prevent premature closure of the wounds. Trephine holes should be at least 2.0 to 2.5 cm in diameter or they will close prematurely. Liquid pus is a positive prognostic sign, and pyogranulomatous or solid tissue in the sinus is a grave prognostic sign. Antibiotic selection must be based on culture and susceptibility testing and should be continued for 2 to 4 weeks. Analgesics such as oral aspirin are used to improve the patient’s comfort.
Prognosis is fair to good with appropriate therapy as described above unless neurologic signs have been observed. Neurologic signs and orbital cellulitis constitute severe and usually fatal complications of chronic frontal sinusitis. On several occasions, especially in animals less than 18 months of age, Dr. Rebhun performed enucleation successfully to allow orbital drainage necessitated by severe orbital cellulitis and ocular proptosis in addition to trephination of the affected sinus. Long-term wound care, antibiotics, and nursing are essential if treatment is elected for such complicated cases.
Necrotic Laryngitis (Calf Diphtheria)
Etiology and Signs.
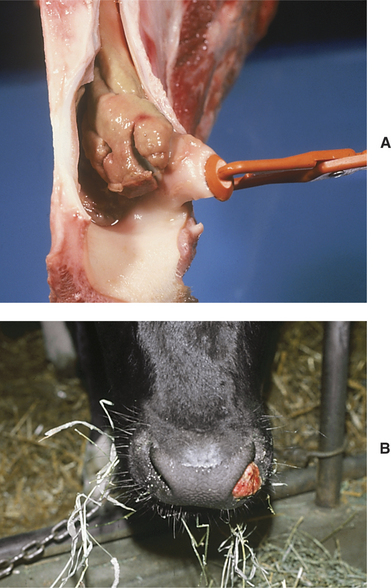
Necrotic laryngitis represents an atypical site of infection by the anaerobe Fusobacterium necrophorum, the organism responsible for calf diphtheria. Calf diphtheria is an infection of the soft tissue in the oral cavity following mucosal injury caused by sharp teeth in calves of 1 to 4 months of age. Calves affected with calf diphtheria usually have abscesses in the cheek region, have mild salivation, and may refuse solid feed (Figure 4-12). The infection spreads among calves fed from common utensils or those in such close contact that they may lick one another. When the larynx becomes infected in the atypical form of this disease, the affected calf develops a progressive inspiratory dyspnea. Low-grade fever (103.0 to 104.5° F/39.44 to 40.28° C) may be present along with a painful short cough that is observed when the calf attempts to drink or eat. As the condition worsens over several days, both inspiratory and expiratory dyspnea may be apparent, but the inspiratory component always will be worse. A necrotic odor may be present on the breath.
Diagnosis.
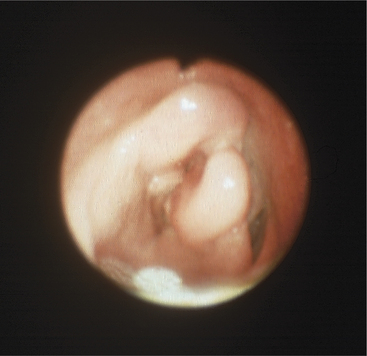
Endoscopy is helpful in confirming the diagnosis. In some calves, the lesions can be seen by using an oral speculum, but endoscopy is much easier and less stressful for the patient. If the calf is in extreme dyspnea or is anoxic or cyanotic, a tracheostomy should be performed before endoscopy. The larynx will be found to be uniformly swollen and may appear to have cartilaginous deformities in chronic cases (Figure 4-13). The laryngeal opening always is narrowed, and mucosal necrosis will be present in acute cases. Chronic cases may have laryngeal deformity and airway narrowing, but the necrotic, infected cartilage may be covered by normal mucosa (see video clips 6 to 8).
Tracheal Obstruction
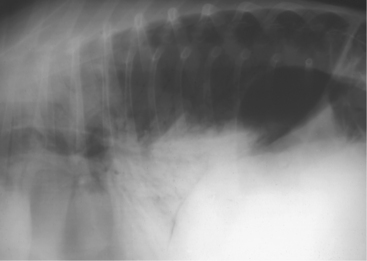
Tracheal obstruction is not common but may occur from either intraluminal obstruction such as infectious bovine rhinotracheitis (IBR) infection or from extraluminal obstruction caused by abscess or lymphosarcoma or as a result of proliferative callus on the first ribs in calves (Figure 4-14). Congenital tracheal stenosis independent of rib injury has also been reported to occur within the cervical or thoracic portions of the trachea.
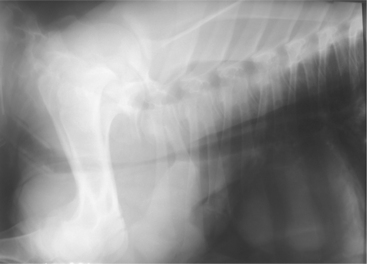
Figure 4-14 Radiograph of a 2-month-old calf with tracheal compression caused by callus formation on the first rib.
Diagnosis is generally easy if endoscopy and radiographs can be used to support the clinical examination. Most calves with tracheal obstruction resulting from proliferative rib callus are several weeks of age when respiratory signs develop and have a history of dystocia at birth.
DISEASES OF THE LOWER AIRWAY
Bacterial Bronchopneumonia
Mannheimia haemolytica
Etiology and Signs.
A great deal of variation in pathogenicity and antibiotic resistance exists for various isolates of M. haemolytica. Therefore the veterinarian must accept the fact that signs produced by these types will vary from mild to severe. Mild infections or less pathogenic M. haemolytica may mimic P. multocida with respect to clinical signs and response to therapy, whereas severe infections may be so drastic as to cause death within hours of the first clinical signs. In rare instances the death can be so peracute that toxicity is expected. A less pathogenic form has been seen causing high fever in recently fresh cows, all of which had a remarkably quick recovery following treatment with ceftiofur.
Signs of acute M. haemolytica pneumonia include fever, depression, anorexia, markedly decreased milk production, salivation, nasal discharge, moist painful cough, and rapid respirations (Figure 4-15). The fever may be as high as 108.0° F (42.22° C) but usually ranges between 104.0 and 107.0° F (40.0 to 41.67° C). Auscultation of the lungs reveals moist or dry rales in the anterior ventral lung fields bilaterally. Bronchial tones indicative of consolidation in the ventral lung fields are observed much more frequently than with acute P. multocida infections (Figure 4-16). Pleuritic friction sounds may be ausculted in some cases because of stretching or compression of fibrinous adhesions between the parietal and visceral pleura. The dorsal lung fields may sound normal on auscultation of animals with mild to moderate M. haemolytica pneumonia. In more severe cases, however, the dorsal lung may be forced to overwork because of the ventral lung consolidation. This overwork creates interstitial edema or bullous emphysema on occasion, and these pathologic changes cause the dorsal lung to be abnormally quiet on auscultation. Auscultation of the trachea will reveal coarse rattling or bubbling sounds caused by the inflammatory exudate free in the trachea. Palpation of the intercostal regions over the pneumonic lung causes the animal pain. Occasional cases will have an accumulation of transudative or exudative pleural fluid in the ventral thorax unilaterally or bilaterally that will cause a total absence of sounds when auscultation is performed.
More severe or neglected cases may show open mouth breathing (Figure 4-17), anxious expression, subcutaneous emphysema secondary to tracking of air from bullae rupture in the dorsal lung field, and have harsh bronchial tones ventrally with inaudible lung sounds dorsally. Respiratory dyspnea is marked in such cases and affects both inspiratory and expiratory components, with the expiratory component being the most obvious. An audible grunt or groan may accompany each expiratory effort, and the animals are reluctant to move because of hypoxia and painful pleuritis.
A peracute rapidly consolidating form of M. haemolytica bronchopneumonia occasionally has been observed over the past 10 years in the northeastern United States and has resulted in high morbidity and mortality within affected herds. The causative M. haemolytica has proven extremely resistant to antibiotics. In some instances, it was resistant to all antibiotics approved for use in dairy cows. Signs in acutely affected cattle include high fever (106.0 to 108.0° F/41.11 to 42.22° C), marked depression, salivation, increased respiratory rate (60 to 120 breaths/min), complete anorexia and milk cessation, reluctance to move, absence of rales when the ventral lungs are ausculted, profound bronchial tones bilaterally that indicate consolidation of 25% to 75% of the ventral pulmonary parenchyma (Figure 4-18), and quiet or inaudible sounds in the dorsal lungs where the remaining pulmonary tissue has been subjected to extreme mechanical and physiologic stress to maintain gas exchange. Subcutaneous emphysema and pulmonary edema are common sequelae in these cattle. Ventral abdominal pain can be elicited in the cranial abdomen as a result of the fibrinous pleuritis present. This pain and absence of rumen activity coupled with the other signs have caused many veterinarians to confuse the initial case of rapidly consolidating pneumonia as peritonitis caused by hardware or perforating abomasal ulcer. The major reason for this error is the absence of rales with this form of M. haemolytica. Therefore we have had to “retrain” our ears to auscult carefully for bronchial tones versus normal or harsh vesicular sounds. Careless auscultation of air sounds in the ventral lung field may not discriminate between bronchial tones and vesicular sounds. Acute infection with this form of M. haemolytica will result in progressive dyspnea and death in 12 to 48 hours unless the veterinarian is fortunate enough to choose as the first treatment an antibiotic to which the organism is susceptible.
Diagnosis.
As with P. multocida pneumonia, accurate diagnosis of M. haemolytica bronchopneumonia requires culture of the organisms from tracheal wash specimens collected from acute, untreated cattle (Figure 4-19) or postmortem cultures of lung and lymph node specimens. Because mortality is greater for M. haemolytica than P. multocida, autopsy specimens often are the source of diagnostic material.
Gross pathology specimens show a bilateral fibrinous bronchopneumonia with 25% to 75% or more of the lungs involved. The distribution is anterior ventral in all cases, and the affected lung is firm, meaty, friable, and discolored. Usually fibrin is present on both the visceral and parietal pleura. Increased amounts of yellow or yellow-red pleural fluid are found frequently. In acute cases with advanced pulmonary parenchymal consolidation or in chronic cases, the dorsal lung may have bullous emphysema or interstitial edema present.
Treatment.
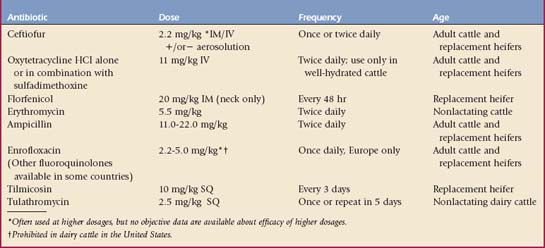
Broad-spectrum antibiotics constitute the major therapeutic defenses against M. haemolytica pneumonia. Once again, the veterinarian is forced to use “best guess” judgment when selecting an initial antibiotic in such cases. Following collection of appropriate diagnostic samples, antibiotic therapy should commence immediately. Because life-threatening signs usually appear in at least some of the affected cattle, the veterinarian is more likely to select broad-spectrum antibiotics immediately. The current popular antibiotics for cows and calves are shown in Table 4-1. Even when the causative bacterial organism is known, antibiotic therapy may be unable to cure the patient for a variety of reasons, such as the chosen antibiotic does not reach adequate tissue levels in the lung; the organism is resistant to the antibiotic; the organism is sensitive in vitro but in vitro inhibitory concentrations do not occur in the cow as a result of the dose, frequency of dosage, or other pharmacologic considerations; the drug may not be able to penetrate consolidated tissue or work in purulent tissue; and in vitro susceptibility tests may not reflect in vivo success of an antibiotic against a specific organism—thus the Kirby-Bauer disc assay has been criticized as too gross compared with mean inhibitory or bactericidal concentration tests that can give a concentration of drug that inhibits or kills an organism. This mean inhibitory concentration then can be compared with known achievable blood and tissue levels of the antibiotic in the cow to determine likelihood of successful treatment. The pathology may be irreversible or viral, and Mycoplasma or A. pyogenes pathogens may coexist to complicate the treatment plan. Textbook charts that quote percentages of isolates sensitive to various antibiotics are seldom helpful because both geographic differences in strains and temporal resistance patterns occur. Appropriate withdrawal times for any antibiotic selected for milk and slaughter residues must be known and observed and may shape decisions by the producer as to which antibiotic is chosen so that an immediate slaughter option is maintained.
The industry continues to seek the “silver bullet”—a magic antibiotic that will cure all cases of Mannheimia pneumonia. This silver bullet would take away the need for diagnostic work or preventive medicine, excuse management techniques that predispose to pneumonia, and of course would only be available through veterinarians. As a profession, we persist in overuse of every new antibiotic that becomes available. We ask these antibiotics to do things that cannot be done while ignoring older time-tested antibiotics. The silver bullet does not and will not exist.
Pasteurella multocida
Etiology and Signs.
The signs of acute P. multocida pneumonia include fever, depression, mild to severe anorexia, moist cough, increased rate and depth of respiration, and a decrease in milk production commensurate with the degree of anorexia. The fever ranges from 103.5 to 105.5° F (39.72 to 40.83° C) in most cases. Moist and dry rales will be ausculted in the anterior ventral lung field bilaterally and are classical findings in acute cases. Usually the dorsal lung fields are normal. Nasal discharge may be serous or mucopurulent in nature and is more apparent in calves than adult cows. The acute disease may occur in calves and cows of any age but tends to be more common in weaned calves and other grouped animals. When seen in younger animals, the acute disease usually is indicative of poor ventilation, excessive ammonia fumes, failure of passive transfer of immunoglobulins, and/or part of a diarrhea/pneumonia complex. All these predisposing factors are common in dairy calves placed in veal operations or other indoor group housing facilities. P. multocida has been found as the cause of neonatal septicemia in calves receiving inadequate colostrum. These septicemic calves may show signs of meningitis, septic uveitis, septic arthritis, and mucopurulent nasal and ocular discharge (Figure 4-20) in addition to the typical signs of acute P. multocida pneumonia.
Acute P. multocida pneumonia tends to occur as either an infectious epidemic or endemic disease in groups of housed calves or adult cattle and may affect 10% to 50% of the animals within a group. It is one of the causes of “enzootic pneumonia” in calves, but this is not the preferred term because it gives little information as to the exact cause of pneumonia. During an acute outbreak, the degree of apparent illness and auscultable degree of pneumonia will vary greatly among affected cattle or calves. If only one animal in a group is infected, predisposing causes or stress unique to that animal should be sought when establishing a history (e.g., recent purchase, recent calving, possibility of BVDV-persistent infection [Figure 4-21], transport to a show, sale, or poor ventilatory management).
Chronic pneumonia resulting from P. multocida causes signs similar to the acute disease, but bronchial tones indicative of consolidation frequently are limited to the anterior ventral lung fields. The abnormal area may be missed unless the stethoscope is pushed under the shoulder and the calf or cow forced to take a deep breath. In calves this can be accomplished most easily by holding the mouth and nose shut for a short period (Figure 4-22). Animals affected with chronic pneumonia may have marked exacerbation of dyspnea and an increased respiratory rate ($60 breaths/min) if housed in poorly ventilated areas or where the environmental temperature exceeds 70.0° F (21.1° C). A. pyogenes is a common secondary invader in lungs chronically infected with P. multocida. Following acute epidemic P. multocida pneumonia, occasional affected animals may show signs of chronic pneumonia.
Diagnosis.
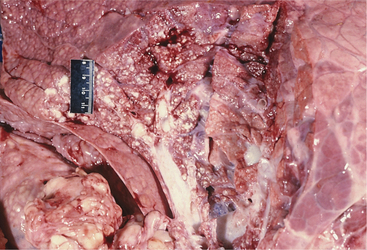
Gross pathology of fatal acute cases includes bilateral anterior ventral pneumonia with the affected portion of lung being firm and discolored red or blue (Figure 4-23). Palpation of the firm affected lung is the key to gross pathologic diagnosis. Fibrin may coat the surface of the parietal or visceral pleura but tends to be less than that observed with M. haemolytica. Chronic cases will show similar firm, pneumonic lung parenchyma but often have bronchiectasis and pulmonary abscesses.
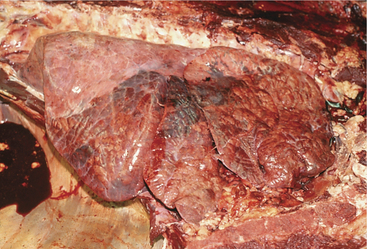
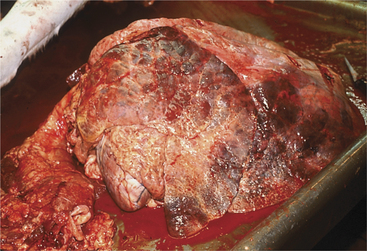
Figure 4-23 Necropsy findings in a calf that was affected with severe cranioventral pneumonia caused by P. multocida.
Treatment.
Penicillin, tetracycline, florfenicol, ampicillin, or ceftiofur may be selected for initial therapy. Dosages and frequency of administration are listed in Table 4-1. Regardless of the antibiotic selected, all treated cattle should have temperature and attitudes recorded daily so that 24- and 48-hour evaluations can be assessed. A trend of decreasing temperature into the normal range should proceed at 1 to 2° F per day when an effective antibiotic is used; the attitude, appetite, and degree of dyspnea should improve along with the return to normal body temperature. Hjerpe has done extensive work in feedlot cattle to estimate probable efficacies of various antibiotics in pneumonia outbreaks. This material is an excellent reference, but the veterinarian must remember that geographic variations in bacterial serotypes and antibiotic susceptibility exist and that antibiotic resistance is likely to increase in years to come. Individual treatment generally is easier for dairy animals than beef animals. Antibiotics such as tetracycline, sulfa drugs, and tylosin have been added to feed and water to treat large groups of calves or heifers. This method may be utilized if the animals are not too sick to eat or drink. If affected cattle are completely off feed, this method is ineffective. When faced with an obvious epidemic, the veterinarian may choose to divide the animals requiring treatment into three groups—each group consisting of animals with mild, moderate, and severe signs. Each group then could be treated with a different antibiotic. Twenty-four hours after initial treatment, each group would be evaluated for relative degrees of improvement and all sick animals given the antibiotic that resulted in the most improved group.
The recognition and correction of management problems or ventilation deficiencies may be as important, if not more so, than any of the previous pharmaceuticals when treating endemic P. multocida pneumonia. Because the organism primarily is an opportunist that gains access to the lower airway following insults to the physical, cellular, or secretory defense mechanisms, predisposing causes should be sought and corrected. In calves, poor ventilation, crowding, and poor husbandry relating to excessive ammonia fumes may be sufficient to allow P. multocida to descend from its normal habitat of the upper airway and colonize the lungs. Examples include changeable temperature and humidity when calves are grouped during the indoor housing season (especially fall, spring, and during winter thaws), broken fans, failure to clean large pens when calves have been in groups for weeks to months, lungworms, and drafts that the confined calves cannot escape. Fresh air is vital to recovery and should be provided even if it means allowing the animals access to outside air in inclement weather.
Vaccinations are included in the prevention section and are discussed on pages 107-109.
Histophilus somni
Diagnosis.
Because the signs usually are identical to those of Pasteurella pneumonia, the veterinarian should collect appropriate samples (tracheal washes for culture and bacterial sensitivities or autopsy cultures from lung and lymph nodes) and institute therapy. A failure of response to standard broad-spectrum antibacterial therapy typifies H. somni pneumonia. Usually an exact diagnosis as to etiology has to await culture and sensitivity results from diagnostic samples. CBCs are variable and nonspecific, with either a degenerative or regenerative left shift observed and elevated fibrinogen levels. Acute and convalescent serum may be helpful retrospectively if the diagnostic laboratory utilized for testing has the capability to establish H. somni titers.
Arcanobacterium pyogenes Chronic Suppurative Pneumonia
Etiology and Signs.
Signs are indicative of chronic or recurrent infection, the hallmark of A. pyogenes pneumonia. The history usually indicates illness of at least 1 week’s duration or recurrent episodes of pneumonia over weeks to months. There may only be one (usually adult cattle) or a few animals (usually calves) affected out of a group or herd. In adult dairy cattle, it is common for clinical signs to develop following freshening (Figure 4-24). In some cases, there may be severe subcutaneous emphysema over the dorsum, suggesting a rupture of diseased alveoli associated with calving as a cause of the pneumomediastinum, subcutaneous emphysema, and sometimes pneumothorax. Although this should be considered in cattle with dorsal emphysema following calving, similar emphysema may be found sometimes in apparently healthy cattle following calving and of course in cattle with interstitial pneumonia. Affected animals may show low-grade fever (103.0 to 105.0° F/39.44 to 40.56° C), rapid respiratory rate (40 to 100 breaths/min), dyspnea characterized by exaggerated inspiratory and especially expiratory efforts (particularly when stressed), head and neck extension when lying down, cough, nasal discharge (Figure 4-25), rough hair coat, poor body condition, depression, inappetence, or decreased milk production. Some cattle maintain normal respiratory rates but exhibit the other signs. Chronic suppurative pneumonia should always be considered a differential for the “poor doing” cow. Auscultation of the lungs reveals moist and dry rales in the ventral 25% to 50% of both lungs in calves and one or both lungs in adult cattle, bronchial tones indicative of consolidation in the ventral lung fields, and coarse tracheal rales caused by a thick mucopurulent airway exudate. High environmental temperatures, high humidity, and poor ventilation exacerbate the clinical signs. A fetid smell may be present following a cough if anaerobic bacteria are present. Auscultation during rebreathing, paying close attention to the cranioventral lung fields under the triceps musculature for the presence of bronchial tones indicative of consolidation, is important when investigating possible cases of mild to moderate chronic suppurative bronchopneumonia.
Diagnosis.
Radiographs or ultrasonography of the thorax is helpful in establishing a prognosis because lung abscesses, bronchiectasis, and consolidation (sometimes remarkably severe in a single lobe) (Figure 4-26) are common in the affected lung (see video clip 9).
Figure 4-26 Radiograph of a cow with chronic suppurative pneumonia and a dramatic lobar consolidation.
A CBC may show neutrophilia or be normal. Serum globulin often is in the high range of normal or elevated ($5.0 g/dl), especially in adult cattle. The animal should be screened for persistent infection with BVDV via buffy coat viral isolation. Gross autopsy of fatal cases reveals anterior ventral consolidation with areas of purulent bronchiectasis and multiple pulmonary abscesses (Figure 4-27).
Treatment.
Treatment is frustrating, and the prognosis is poor for pneumonia caused by A. pyogenes. Other causative organisms such as P. multocida, M. haemolytica, Mycoplasma, and/or Fusobacterium also may be cultured from the tracheal wash sample. Penicillin is the drug of choice and should be given at 22,000 U/kg twice daily for 7 to 30 days. Although penicillin is effective against A. pyogenes in vitro, the pulmonary in vivo infection should be likened to an abscess because of the heavy accumulation of A. pyogenes pus in areas of bronchiectasis or encapsulated lung abscesses. If another pathogen, in addition to A. pyogenes, is isolated from the tracheal wash sample, appropriate antibiotic therapy should be selected for this organism as well. Ceftiofur, ampicillin, and tetracyclines are other commonly used therapies. Clinical treatment frequently results in short-term improvement followed by relapse when the animal is stressed or subjected to high environmental temperatures, humidity, or poor ventilation. Signs of improvement will be indicated by normal rectal temperature, improved respiratory function, and improvement in overall body condition and attitude. Many affected animals eventually succumb to the infection or are culled because of poor condition and production.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree