Reproductive Surgery
Female
Ovariectomy and Ovariohysterectomy
Ovariectomy (OVX) and ovariohysterectomy (OHX) are done for the purpose of elimination of females from breeding programs, for elimination of undesirable genetics (poor textile traits, genetic defects), and because of acquired diseases. Acquired diseases of the ovary include ovarian teratoma, ovarian granulosa theca cell tumors, interstitial cell tumor, and ovarian or paraovarian cysts (Figure 62-1).1,2 Diseases of the uterus may include segmental aplasia, chronic infection, and uterine neoplasia.3 OVX and OHX may be done via traditional laparotomy or by laparoscopy. Laparoscopy is the preferred technique for ovariectomy, especially unilateral OVX. Laparoscopic OHX is done infrequently. Laparotomy may be done for OVX or OHX and most often is accomplished with the camelid under general anesthesia. Unilateral OVX may be done via lateral abdominal (flank) laparotomy or caudal ventral midline laparotomy. Bilateral OVX and OHX are most easily done via caudal ventral midline laparotomy, with the patient under general anesthesia and placed in the Trendelenburg position (dorsal recumbency, head and neck tilted downward at a 30-degree declination).
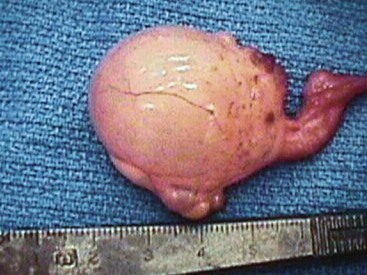
Figure 62-1 Right ovary of an adult female alpaca presented for chronic nonreceptivity and failure to become pregnant. The right ovary is enlarged and was removed. An ovarian teratoma was diagnosed via histopathology.
Uterine Torsion
Birthing in llamas and alpacas is a rapid process. Studies in South America documented that greater than 80% of crias (neonatal llamas or alpacas) are born between 6 AM and 1 PM.1 Stage II labor (expulsion of the cria) occurs over a period of 10 to 15 minutes (range 6–47 minutes).4 Dystocia is an uncommon event in llamas and alpacas. Studies in South America found that dystocia in alpacas (1660 birthings observed) occurred in 1.6% of birthings and that 25% of these occurred in primiparous females. Data in a smaller number of llamas (234 birthings observed) demonstrated dystocia in only 1 female (0.4%).4 Interestingly, causes of dystocia differ between South American and North American camelids. Uterine torsion is rarely found in descriptions of dystocia in South American camelid herds. Causes of dystocia in these populations include fetal malpositioning, with 30% of those occurring with the fetus in a posterior presentation and 70% in anterior presentation.4 Markedly less information is available documenting causes of dystocia in North American herds.5,6 The available data seem to indicate that uterine torsion is a common cause for veterinary intervention of dystocia.5,7 Our opinion is that fetal malpositioning is associated with the majority of dystocia in llamas and alpacas and that uterine torsion is overrepresented in the literature because these cases are more likely to be presented to teaching hospitals for treatment.6
Uterine torsion occurs when the pregnant uterine horn rotates around the long axis of the uterus and remains in that position. In nearly all cases of diagnosed uterine torsion, the veterinary examination of the female is performed because of abnormal dam behavior or clinical signs of abdominal pain noted by the owner. Uterine torsion was reported to account for 60% of dystocia cases presented to a veterinary teaching hospital and has been reported to cause 30% of dystocias in dromedary camels in another institution.5,8 It is likely that uterine torsion may occur and resolve without clinical signs being noted during early stages of pregnancy. As pregnancy advances, torsion of the uterus is less likely to self-correct. Therefore, females having clinical signs associated with uterine torsion should be treated as an emergency to save the viability of the fetus and the life of the dam. Delays in treatment may increase the risk of strangulation or rupture of the uterus and rupture of the uterine artery.
Most clinical cases of uterine torsion occur after the ninth month of pregnancy. Unlike horses and cattle, in which uterine torsion most often occurs at term, uterine torsion in alpacas and llamas frequently occurs 2 to 6 weeks before the due date for parturition. In a report of 20 uterine torsions in llamas and alpacas, 90% of the affected females were in greater than 335 days of pregnancy.5 When possible, we leave the fetus to continue to a natural birthing process (e.g., in females having a uterine torsion greater than 2 weeks before parturition and closed cervix). Poor survival of crias born after induction of parturition or premature birth has been reported.9 In that study, induction of parturition was attempted with fluprostenol, oxytocin, or three different dosages of dexamethasone. Alpacas given fluprostenol delivered live crias a mean of 21.5 hours after treatment. Oxytocin in combination with dexamethasone (0.05 milligram per kilogram (mg/kg)) failed to induce parturition. Dexamethasone at 0.5 mg/kg resulted in stillborn fetuses. Thus, neither oxytocin nor dexamethasone can be recommended for induction of parturition in alpacas. We routinely monitor fetal heart rate, placental thickness, and placental fluid echogenicity to assess fetal well-being. Cesarean section is performed if the fetus is determined to be at risk. Ultrasonographic monitoring of the fetus may include echotexture of the fluids within the heart and body cavities of the fetus. Evidence of impending fetal distress include decreasing heart rate, placental fluids developing a turbid appearance on ultrasonography, rapid changes in placental thickness, and apparent separation of the placenta from the endometrium.10,10a
Nonsurgical correction of uterine torsion requires accurate assessment of the direction of the torsion. Incorrect or lack of determination of the direction of torsion before rolling the dam may cause progression of the torsion and obstruction of arterial blood flow to the fetus and uterus. Uterine torsion occurs as either clockwise (torsion of the left horn to the right side) or counter-clockwise (torsion of the right horn to the left side) rotation. The clockwise or counter-clockwise direction of rotation is referenced by the observer standing behind the patient and looking at the rear of the female. The pregnant uterine horn moves around the long axis of the uterus similar to the direction of the rotation of the hands of a clock. When visualizing a clock face with the vulva at the center, the normal, nonpregnant uterine horns are positioned at the 3 o’clock (right horn) and 9 o’clock (left horn) positions. With advancing gestation, the pregnant horn gravitates ventrally and toward midline. This shift in position does not effect the orientation of the broad ligaments of the uterus which remain parallel coursing from their caudodorsal attachments towards a cranioventral position. The broad ligaments become thicker and more taut as the uterus become more dependent with the weight of the fetus and fluids. The caudal extent of uterine torsions is often anterior to the cervix. Thus, vaginal speculum examination may fail to diagnose the presence of a uterine torsion. At term, the presence of a torsion will interfere with cervical dilation. In a report of 20 occurrences of uterine torsions diagnosed in 11 llamas and 3 alpacas, 95% were in a clockwise direction.5 Counter-clockwise uterine torsion may be more common in alpacas than in llamas.10 These differences exemplify the need for accurate diagnosis of the direction of uterine torsion before attempting nonsurgical correction.
A variety of risk factors have been suggested as increasing the likelihood of uterine torsion including large birth weight, male gender, and maternal illness.11 Environmental factors that affect dam behaviors may contribute to risk. Activities thought to increase the risk of torsion include rolling, right horn pregnancy, and prolonged duration of pregnancy.
Manual Correction of Uterine Torsion
Manual correction of uterine torsion can be accomplished by rolling the female to “untwist” the torsion. Uterine torsion most often occurs pre-term, and thus transvaginal correction only can be done when the examiner’s hand can be introduced into the uterus. In a report of 20 uterine torsions diagnosed in 14 llamas and alpacas, 5 were corrected by transvaginal manipulation, and 8 were corrected by rolling of the dam combined with application of external compression on the abdomen.5 Rolling to correct uterine torsion is accomplished by placing the dam in lateral recumbency on the same side as the direction of the torsion. A llama having a clockwise uterine torsion (twist to the right, left horn over top of right horn), would be placed on its right side to begin the procedure. The pregnant horn of the uterus is located and stabilized by transabdominal palpation. The female is rolled over its back to its other side whilst using transabdominal pressure to maintain the position of the fetus. The rolling procedure may be repeated if not initially successful. Rectal examination is done after the rolling procedure to assess correction. Reoccurance of uterine torsion is likely associated with incomplete correction. Surgical correction should be pursued if rolling is not successful.
Surgical Correction of Uterine Torsion
Surgical correction of uterine torsion may be done under clean field conditions. In a case report of 20 uterine torsions, 7 required celiotomy to correct the torsion.5 Laparotomy may be done via the left lateral or ventral midline approaches. General anesthesia is best suited for ventral midline laparotomy. Lateral abdominal wall laparotomy may be done with sedation and local anesthesia, or with general anesthesia. Care must be taken to minimize the total dose of lidocaine used because llamas and alpacas are more susceptible to lidocaine toxicity. A total body dose of 4 mg/kg body weight is suggested as the maximum tolerable dose of lidocaine. We prefer to approach the abdomen from a left-sided laparotomy.10 Care must be taken in this approach because the spleen of llamas and alpacas is positioned in the midportion of the flank region. The spleen may be easily injured during opening of the transversus abdominus muscle and the peritoneum. The site for the lateral incision is made starting at a point 6 to 8 cm cranial and ventral to the tuber coxae and extending 8 to 10 cm cranially and ventrally toward the costochondral junction. The incision is made only large enough for the surgeon to introduce a hand and arm so that the uterus can be corrected in position blindly. Correction of the torsion is confirmed by palpating the broad ligaments, the uterine body, and the fetus. After the torsion has been corrected, the viability of the fetus is assessed. If the fetus is known to be compromised, a 15-cm incision usually will accommodate exteriorization of the uterus for cesarean section. The abdominal muscles and the fascia of llamas and alpacas are thin and have poor holding power for sutures. Careful reconstruction of the abdominal wall with No. 1 or No. 2 synthetic, absorbable suture material having good retention of tensile strength (e.g., polyglactin [PG]-910; polydioxanone [PDS]) is done. Then, skin is closed using No. 1 or No. 2 nylon or polycaprolactam with a continuous interlocking suture pattern. If the fetus is at term (partially open cervix), compromised, or determined to be dead, a cesarean section may be performed at the same time. In rare cases, the uterus cannot be corrected without removal of the fetus. This is a judgment call that the surgeon makes during the procedure. We prefer to leave the fetus in situ if uterine torsion has occurred sufficiently before term posing high risk for survival outside of the uterine environment. In a report of 7 celiotomies done to correct uterine torsion, 6 were done in term females.5 In these 6 females, the fetuses were removed by hysterotomy, of which 4 (66%) survived. In the remaining female, the fetus was left in situ and was delivered stillborn at a later date.
Uterine torsion presents a significant risk to the life of the dam and the fetus. Possible complications of uterine torsion may include fetal death or compromise, premature birth, death of the dam, uterine compromise by ischemia, rupture of the uterine or ovarian artery with hemorrhage, uterine rupture and subsequent peritonitis, and, if surgical correction is necessary, all of the complications associated with laparotomy and cesarean section such as retained placenta, metritis, and adhesions. Of 14 llamas and alpacas with uterine torsion on 20 occasions, nonsurgical correction was successful in 13, and celiotomy was required in 7.5 In these 20 cases, 14 crias were born or delivered alive, and 6 were stillborn. All camelids that had nonsurgical correction of uterine torsion successfully returned to breeding; 5 out of 7 camelids having celiotomy returned to breeding soundness. Careful and detailed evaluation on a regular basis (e.g., every 8 or 12 hours) may allow intervention and emergency delivery of the fetus, if needed. A combination of dam behavior, ultrasonography, fetal heart rate, and fetal cardiotocography is used to monitor the condition of the placenta and the fetus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


