Following conception, the developing embryo descends into the uterus 5–6 days post ovulation. At or around day 36, implantation of the girdle cells of the trophoblast occurs within the uterine mucosa, usually at the base of a uterine horn. These cells create the endometrial cups (see Fig. 12.21) which produce equine chorionic gonadotrophin (ECG). ECG has and ovulatory and luteinizing effect on the follicles present in the ovaries which form secondary and accesory corpora lutea. There is wide variation in their number between different mares. In the majority of mares regression of the endometrial cups occurs by day 150 of gestation, however cases of retained endometrial cups for up to 2 years post foaling have been documented. Both the primary and supplemental corpus luteum begin to regress and are usually completely atrophic by about 210–220 days. From this stage forward, the ovaries of pregnant mares show little follicular activity. • These mares have a female phenotype but a male karyotype. They can display a wide range of physical appearances. Some mares have been noted to be of normal or even large size. • For XY gonadal dysgenesis there is a failure of formation of active gonads. The inactive gonads are comprised of primarily fibrous tissue. • A flaccid cervix and uterus are present with normal external genitalia. Serum testosterone levels are minimal. Androgen insensitivity syndrome or testicular feminization is where tissues are completely or partially insensitive to androgens. This deficiency lies in the androgen receptor gene on the X chromosome which affects responsiveness or sensitivity of the fetus’s body tissues to androgens. Most commonly the external genitalia are female with the cervix and uterus lacking. Testes in the inguinal canal labia or abdomen are present with normal or increased testosterone production. With partial resistance both male and female physical characteristics may be present with stallion-like behavior observed. These rare disorders include deletions, duplications or rearrangements of the autosomal chromosomes. • Karyotyping is a biological means of determining the gene status of that individual with the ability to identify abnormalities. Aseptic peripheral blood lymphocytes are cultured for 64–72 hours and then processed to eventually allow electron microscopic examination and comparison of 31 paired chromosomes plus one pair of sex chromosomes; differences are determined and so allow the identification of chromosomal abnormalities. • Abnormalities of the gonads where intersexuality occurs may be classified according to their morphology. True hermaphrodites have one or both gonads containing both ovarian and testicular tissue, or may have one male and one female gonad. Pseudo-hermaphrodites have the gonads of one sex and the accessory genitalia of the other. A female hermaphrodite has ovaries and male accessory reproductive organs (and the male hermaphrodite the reverse). Ovarian tissue may be extremely small or even absent. Predominant external features of equine hermaphrodites are ventral displacement of the vulva with an enlarged clitoris or a short, backward-projecting penis. Testes may be present with mammary tissue and teats. Testes, epididymis and vesicular glands are usually present abdominally as well as a poorly developed uterus. The genetic nature of this abnormality is uncertain but recorded cases indicate that individual stallions may sire intersex foals from different mares. • Ultrasonographic monitoring of the progression of the dominant follicle should be done during estrus. • Initial indication of a problem may be the thickening of the follicle wall or the detection of echogenic particles within the follicular fluid, potentially progressing to fibrous strands traversing the lumen or the homogeneous appearance of hemorrhage. • Some follicles may luteinize and produce low levels of progesterone while others do not and may persist for long periods of time. Uterine edema will usually subside and the mare behaviorally will be neither receptive nor unreceptive. • If the anovulatory follicle luteinizes then prostaglandin therapy will result in lysis and follicular regression. However, in the absence of progesterone and luteinization, initiating estrus will depend on when the regression of the anovulatory follicle occurs. • A subsequent follicular wave may be initiated in the presence of a regressing anovulatory follicle with behavioral estrus, uterine edema and subsequent ovulation of the new dominant follicle. • Human chorionic gonadotrophin (HCG) and deslorelin (a GnRH agonist) are generally not effective in inducing ovulation of the affected follicle. • Rectal palpation and ultrasound help to identify the size of the ovaries and the follicular activity. • Presence or absence of uterine edema aids in determining if the mare is truly anestrus/transition or coming out of transition. • There are many different drugs and protocols to help induce cyclicity (Table 12.1). Table 12.1 • Ultrasonographic evaluation of the enlarged ovary reveals hyperechogenic homogeneous blood within the lumen of an extremely large follicle, or a multicystic appearance due to fibrin strand formation spanning the diameter of the structure. The affected ovary has a clearly identifiable ovulation fossa on rectal palpation. The contralateral ovary on examination is of normal size and function and mares continue to cycle normally. Endocrine profiles and behavior are normal. • Regression of the hematoma should occur over time, during which a normal interovulatory period will occur. • If the hematoma becomes excessively large it can destroy the remaining normal parenchyma of the ovary and ovarian removal may be necessary because the ovary is left non-functional. • Behavioral changes as described above in combination with lack of an ovulation fossa on rectal palpation, ultrasonographic imaging and serum hormone levels aid in diagnosis. Ultrasonographic appearance of GCTs can vary from enlarged multicystic, honeycomb appearing firm ovaries to a single large cyst or blood-filled structure. • Differentiation between ‘autumn’ or anovulatory follicles and ovarian hematomas is imperative. The contralateral ovary is usually small with no to minimal follicular activity. • Clinical diagnostic assays should include serum antimullerian hormone, inhibin, testosterone and progesterone. These, however, may be equivocal and ascertaining if continued growth with time or regression with hormone therapy (progesterone and estradiol) may be necessary. • Removal of the affected ovary. This can be done via a flank laparotomy, midline or paramedian approach. Return to normal estrous cycles by the contralateral ovary may take 8–12 months. • Cannulation of the oviductal fimbrae via flank laparoscopy in the standing mare, with infusion of fluorescent microsphere beads will allow for the direct visualization of oviduct anatomy and indirect assessment of oviduct patency by performing a uterine lavage at 24 and 48 hours post surgery to identify the microspheres. If the microspheres are not found in the lavage fluids it is assumed the oviduct is not patent. • A ventral midline exploratory laparotomy will also allow for visualization of the ovaries and oviducts for anatomical defects. This may be difficult however in maiden mares whose mesovarians and broad ligaments have not been stretched. Infusion of a sterile 5% new methylene blue dye in normal saline through a catheter into the oviduct and detection of the dye within the uterus will determine patency. • Movement or passage of the blockage through the oviduct can be done using two different techniques, the first of which is flushing the oviducts either via ventral midline laparotomy or standing flank laparoscopy. • The second and less invasive or detrimental to the oviduct is placement of prostaglandin E2 (PGE2) on the surface of the oviduct using a flank laparoscopic approach. The PGE2 potentiates muscular relaxation of the oviduct allowing movement of the oviductal plugs into the uterus. The uterus is comprised of two uterine horns and the uterine body. • Lymphatic cysts can be diagnosed through transrectal palpation, by direct intrauterine palpation via the cervix, through hysteroscopic examination, and endometrial biopsy, but most commonly by transrectal ultrasonography. • The increased use of transrectal ultrasonography has enhanced the practitioner’s ability to differentiate early embryonic vesicles from endometrial cyst. In mares that have a few small cysts, ultrasounding the mare pre or post breeding or ovulation and mapping of endometrial cysts can aid in pregnancy diagnosis by comparing ultrasonographic images. Mares that have numerous clusters or multilobular cysts may need repeat examinations before determination of a pregnancy by increasing vesicular size or a visible heart beat can be established. • Most cysts do not cause a problem, but are usually a sign of an underlying one. These mares are usually older mares with poor uterine clearance and benefit tremendously by simply using uterine lavage and oxytocin in their breeding regimen. Where cysts create a problem is in the distinction or recognition from a pregnancy, however this is more of an inconvenience than a problem. Cyst removal therefore should be delegated to those mares with poor reproductive histories that have large cysts that may obstruct embryo movement or those mares with numerous small cysts that may prevent early embryonic growth or severely compromise the placenta. • Various treatments have been proposed to include: endometrial curettage, puncture by uterine biopsy, aspiration, or lasering during hysteroscopic examination, snare electrocoagulation via hysteroscopy, repeated lavage with warm saline or ablate manually. Large pedunculated cysts are removed when the mare is in heat, by manually snaring the cyst with a gigli wire introduced through a steal double mare catheter. Once the snare is around the base a gradual sawing motion will cut the cyst from the endometrium. This can usually be accomplished without rupturing the cyst with minimal removal or damage to the underlying endometrium. Care must be taken not to remove more than the epithelial lining. A minimal amount of hemorrhage is associated with this procedure and it is recommended to lavage the mare’s uterus for the proceeding couple of days. Smaller more numerous cysts are usually removed via hysteroscopic laser. This technique has not been determined to be satisfactory for large cysts, since so much heat is generated in order to destroy the cyst that there is increased risk of damage to or tearing of the uterus. Smaller cysts, however, are easy to find and destroy. Lasering the cyst at the thinnest section until complete penetration through the wall has been made releases the fluid contents with immediate visible shrinkage. Care must be taken not to laser blood vessels on the cyst’s surface making visibility difficult. • Positive blood serum eCG levels. Care must be taken with some commercial kits since they can cross react with LH. • Transrectal ultrasonography can identify hyperechogenic spots in the base of a horn if the endometrial cups are large and able to be visualized. • Hysteroscopic examination may reveal the circle of peanut-shaped cups in the base of a horn. These will vary in size and color depending on their activity and longevity. • Different treatments have been attempted to help regress existing endometrial cup tissue. These include laser, biopsy and kerosene endometrial curettage. Unfortunately, kerosene is the only treatment to be looked at critically in three mares and it is still questionable whether treatment is to no avail. Regression therefore finally occurs over time. • The persistence of the endometrial cups has been evident in mares up to 2 years post foaling and post 45 day embryonic loss. • Many methods have been used to correct uterine torsion in the mare. The most common are rolling the anesthetized mare and laparotomy via standing flank or ventral midline incision. Although non-surgical methods such as rolling are less expensive, this technique may predispose to separation of the placenta from the endometrium with subsequent abortion, premature birth or death of the fetus. Uterine rupture and tearing are severe complications associated with this procedure. Evaluation of uterine and intestinal compromise is not possible. • Standing flank laparotomy has been more popular than the ventral midline approach and is especially easier earlier in gestation; in addition, general anesthesia is not necessary. The uterus should be palpated for signs of edema, congestion and hematomas. This method carries risks: the mesometrium is under considerable tension, and if the horse is standing during laparotomy, this can lead to perforation of the uterus and injuries to the mesometrium during manual retorsion. • A ventral midline approach allows quick and clear access to the abdominal cavity, visual assessment of uterine wall viability, correction of concomitant gastrointestinal tract problems, and performance of hysterotomy if indicated. Continued monitoring is necessary for systemic compromise and laminitis. Prognosis for fetal survival is good with improvement as gestational age lessens. 1) Middle uterine a arterial rupture usually occurs on the right side of the mare’s uterus, possibly as a result of the cecum displacing the uterus and increasing tension on the right uterine artery in addition to additional direct pressure by the cecum on the artery 2) Normal age-related vascular degeneration 3) Copper deficiency interfering with elasticity of the vessels. Mares with discomfort or colic in the middle or late stages of gestation or 24–72 hours after parturition should be considered to be potential hemorrhage candidates. In some instances, mares are simply found dead because of acute arterial hemorrhage. More often, however, mares develop signs within 24 hours of parturition, and most are associated with rupture of the right uterine artery. Often diagnosis is made on the basis of clinical signs alone. However, it is necessary to determine the extent of hemorrhage so that optimal treatment and prognosis can be established. Transabdominal ultrasound (which can be performed using a 5-MHz linear array rectal transducer) helps to differentiate the swirling of active hemorrhage free in the abdomen from intrauterine bleeding or bleeding within the broad ligament. Filling of the uterine horns with blood can lead to placental separation, and intrauterine trauma may be evident by observation of changes in the fetal fluids during transabdominal ultrasound. To further confirm intra-abdominal hemorrhage versus peritonitis or uterine rupture, abdominocentesis can be performed (see Chapter 1, pp. 34–35). Additional information can be obtained from blood work (with total protein and albumin concentration decreasing prior to a decrease in hematocrit). These findings can help determine which therapeutic regimen to use. • Mares should be treated for shock secondary to hemorrhage, however a fine line is walked between providing enough support to maintain sufficient blood flow to vital organs and avoiding increasing blood pressure to the point of interfering with clot formation and hemostasis. The ultimate treatment has obviously not been found to exist, and many protocols have been reported. The most important initial consideration is to improve perfusion and keep the mare quiet and warm to promote clot formation. Various drugs have been used, including acepromazine, xylazine, butorphanol and diazepam, to keep the mare as comfortable as possible while other treatments are being initiated. Care must be taken when using sedatives since many decrease blood pressure and can further accentuate hypovolemic shock and decrease blood flow to vital organs. With the goal of keeping the mare as quiet as possible, it is probably prudent to leave the foal with the mare, as long as the mare’s movements do not cause harm. If the foal must be removed from the stall, it should be kept in close view of the mare. Further therapy to reverse shock and control hemorrhage can include the following: • Hypertonic saline: the hyper-osmolality of the solution rapidly increases intravascular volume improving perfusion. • Regular isotonic intravenous fluids: provide replacement for intravascular volume expansion, buffering, and dispersal to intercellular space. • Colloids plasma: helps maintain intravascular volume and provides clotting factors; whole blood expands oncotic blood volume while providing increased oxygen carrying capacity, clotting factors and platelets. • Aminocaproic acid: inhibits fibrinolysis via inhibition of plasminogen activator substances and anti-plasminogen activity. • Yunnan baiyao: an oral hemostatic Chinese herbal medicine, used originally by soldiers in the Vietnam war to stop bleeding. • Naloxone: pure opioid antagonist used to improve circulation in refractory shock by binding endogenous opioids hypotension is reduced, cardiac work decreased and pulmonary vascular resistance is lowered. Care should be used when given with butorphenol since they will negate each other. • Formalin: is believed to cross link proteins potentially altering platelet or endothelial cell surface, proteins resulting in activation of platelets or decreased endothelial permeability; recent studies have discourage its use (Sellon, D 1999 AAEP proceedings).
Reproductive disorders
1 The mare
Clinical examination of the mare (Figs. 12.1–12.2)

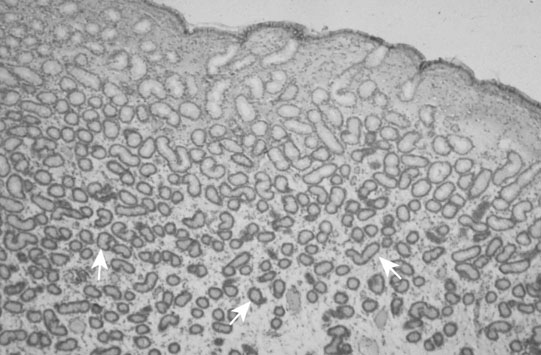
The estrous cycle (Figs. 12.3–12.20)

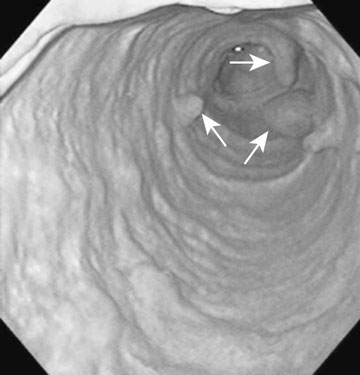
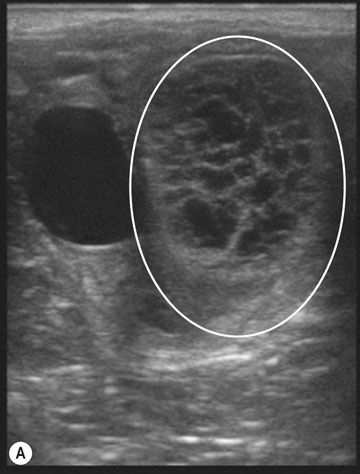
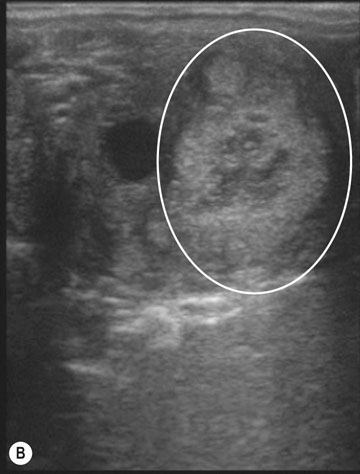
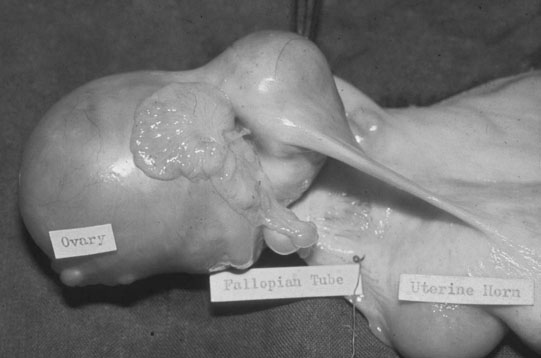
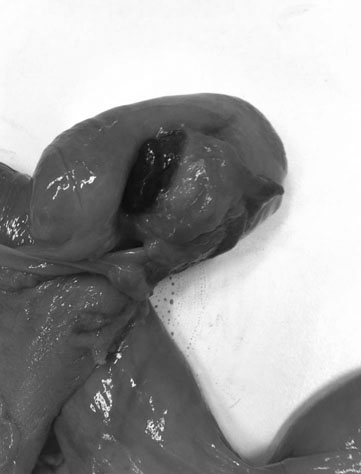
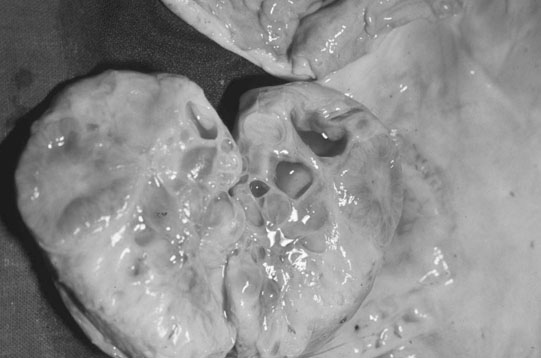
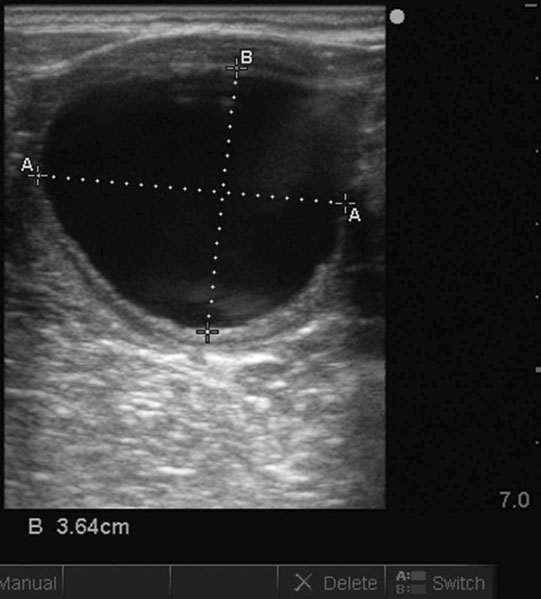
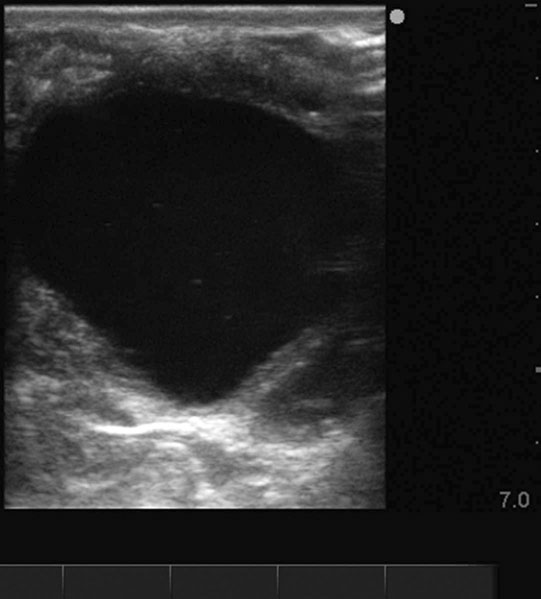
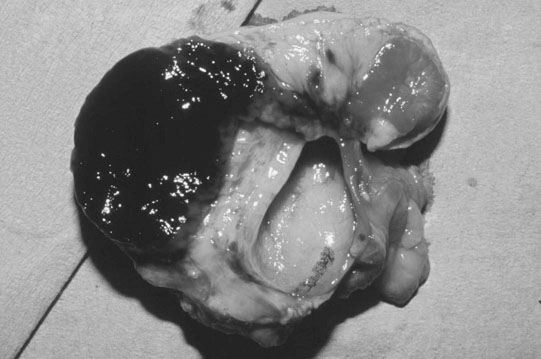
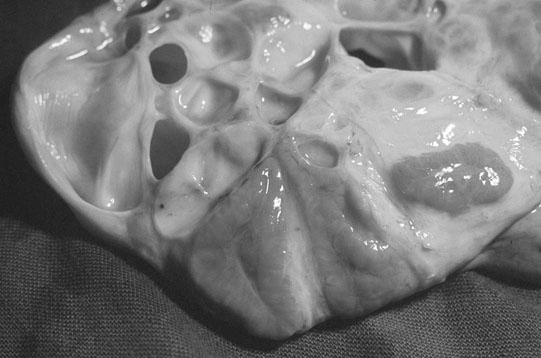
The ovary
Developmental disorders
XY sex-reversal syndrome (64X0)
Clinical signs
Autosomal chromosome abnormalities (Figs. 12.22–12.24)
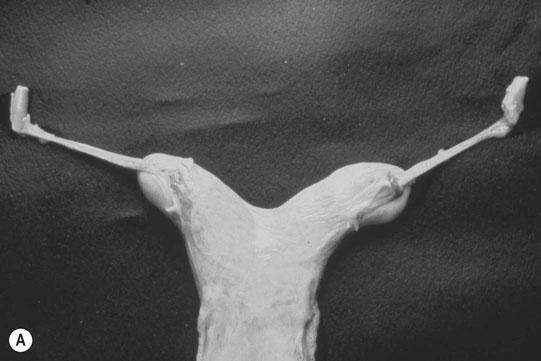

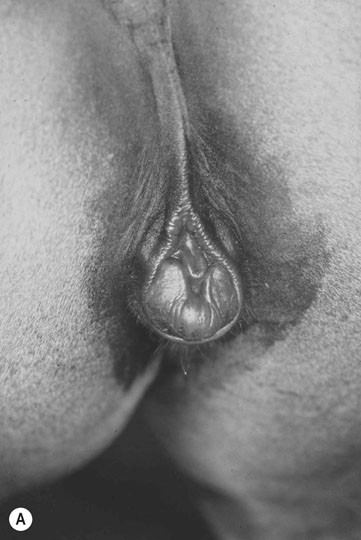
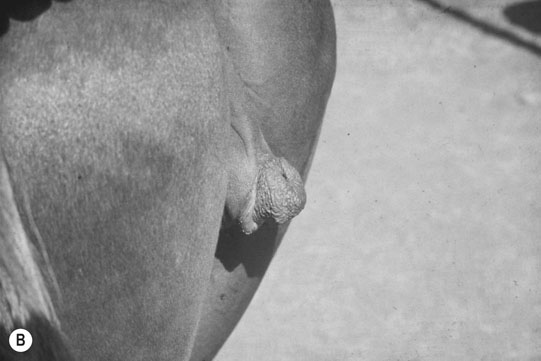
Diagnosis of chromosomal abnormalities
Non-infectious disorders
Anovulatory follicles (Fig. 12.25)
Diagnosis
Treatment
Lack of follicular development (anestrus) (Figs. 12.26 & 12.27)
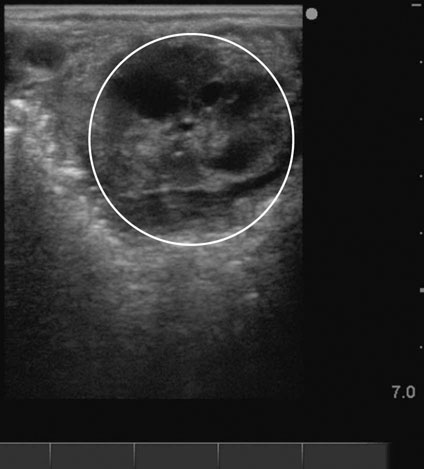
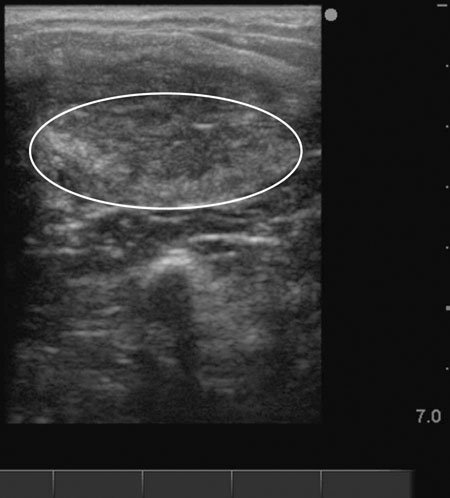
Diagnosis and treatment
Drug
Mechanism of action
Dose
Domperidone
DA2 dopamine receptor antagonist
1.1 mg/kg PO sid–bid
Reserpine
Rauwolfian substance
0.01 mg/kg PO q 24 hour
Sulperide
Selective DA2 dopamine receptor antagonist
3.3 mg/kg/day
Deslorelin
GnRH agonist
62.5 µg IM, q 12 h
Buserelin
GnRH agonist
12.5 µg IM, q 12h
eFSH
FSH
12.5 µg IM, q 12 h until a 35 mm follicle present; then coast for 24 hours, give hCG and breed in another 24 hours
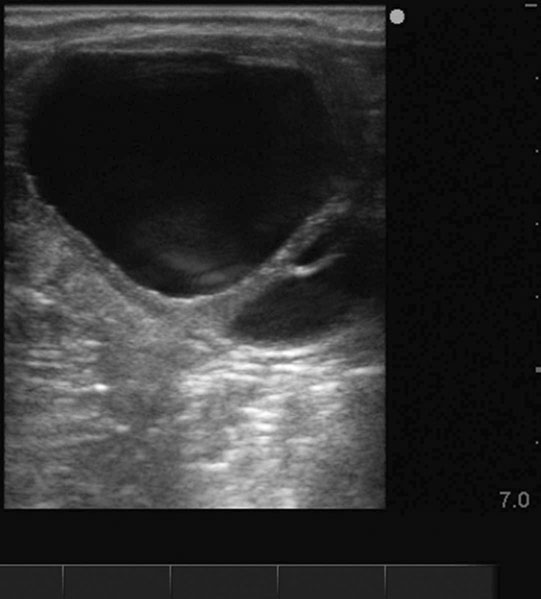
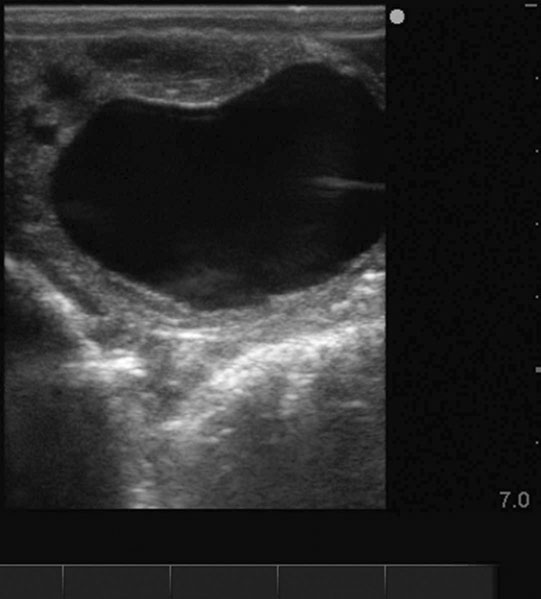
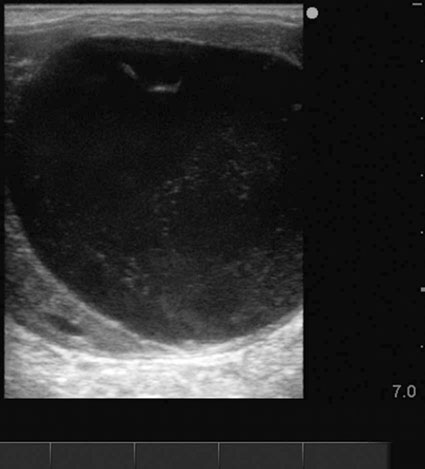
Ovarian hematoma (Figs. 12.28 & 12.29)
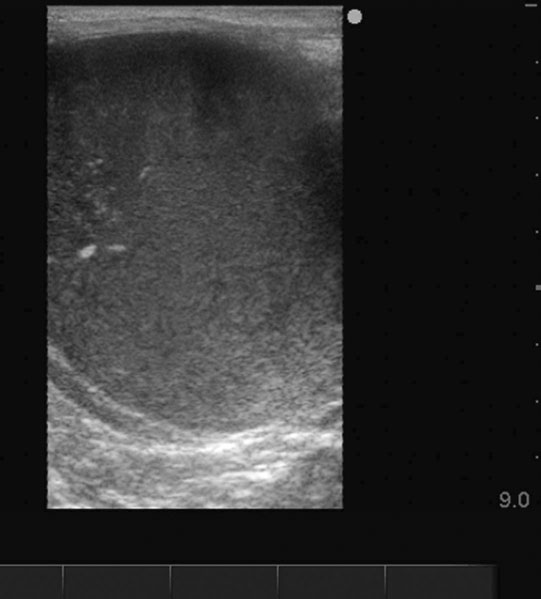
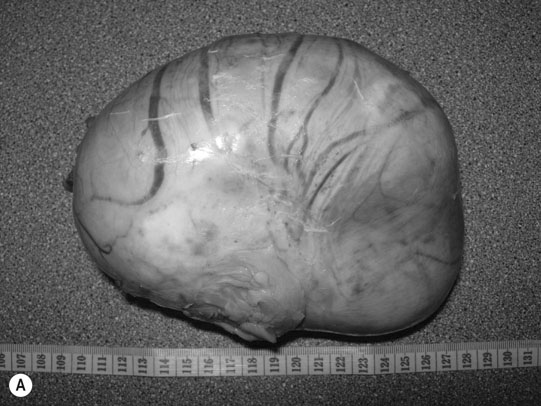
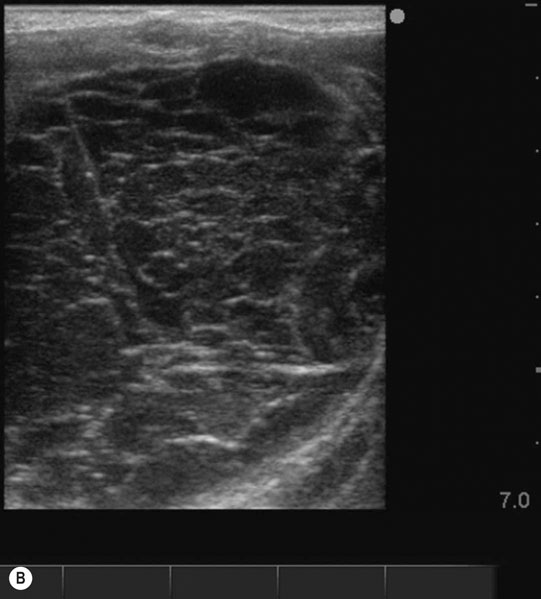
Diagnosis and treatment
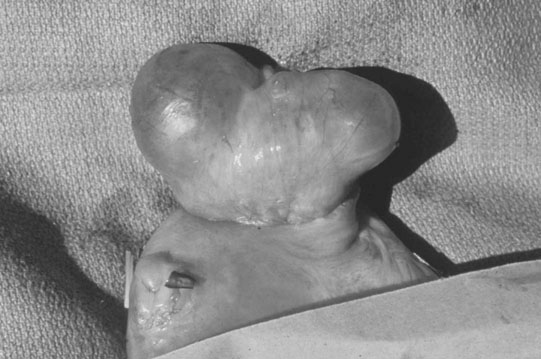
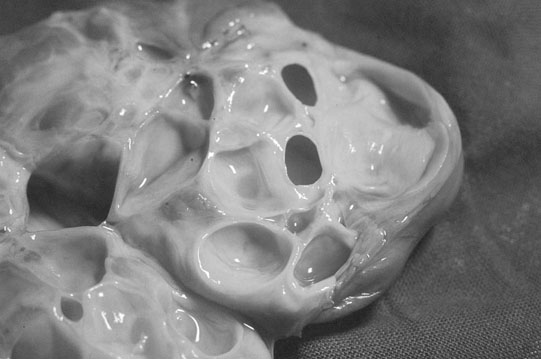
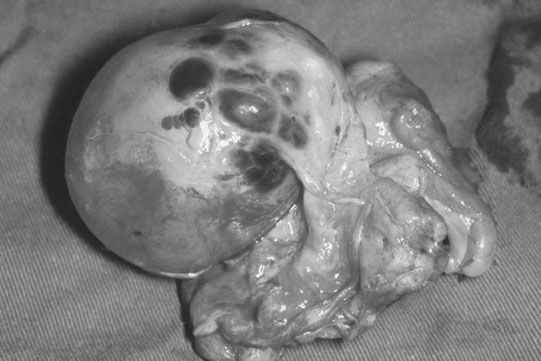
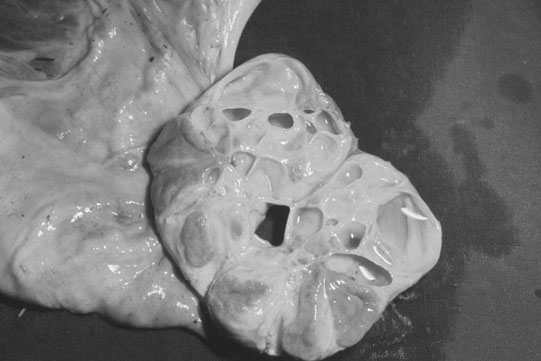
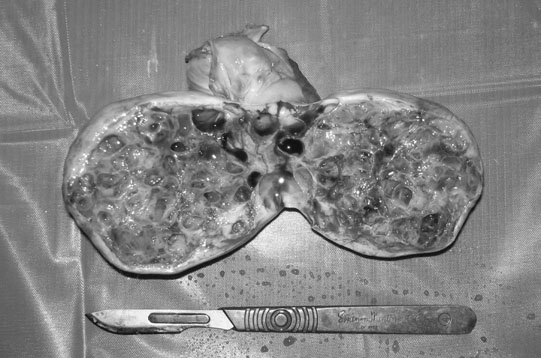
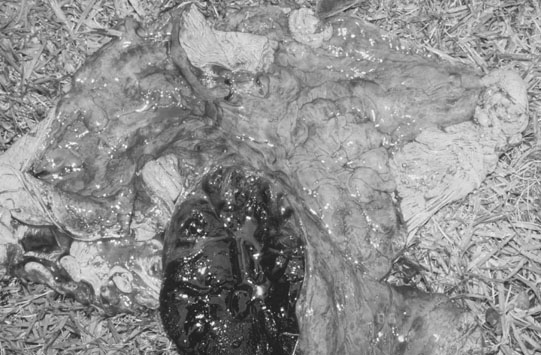
Ovarian tumors (Figs. 12.30 & 12.31)
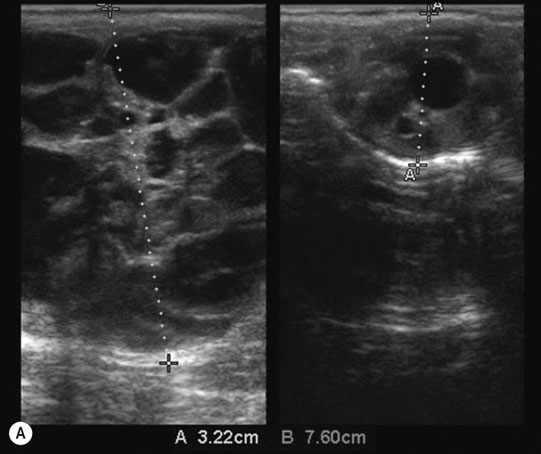
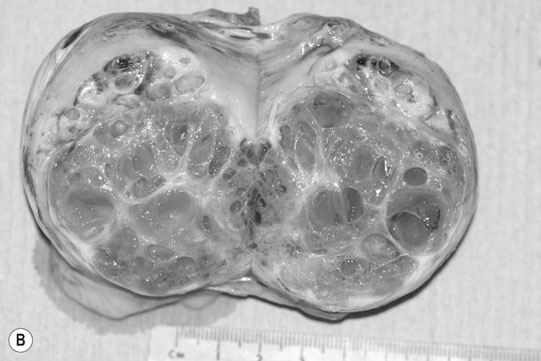
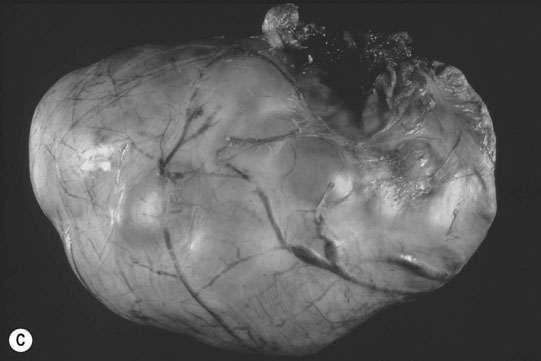
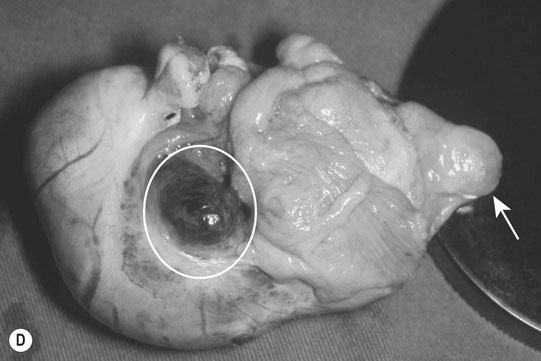
Diagnosis and treatment
The oviduct
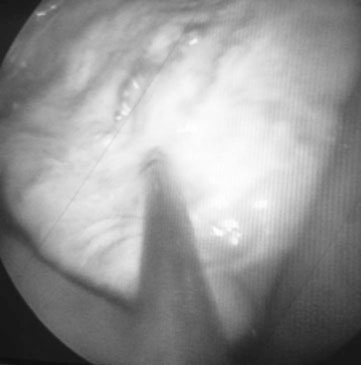
Oviduct blockage (Fig. 12.32)
Diagnosis
Treatment
The uterus
Non-infectious disorders (barren mares)
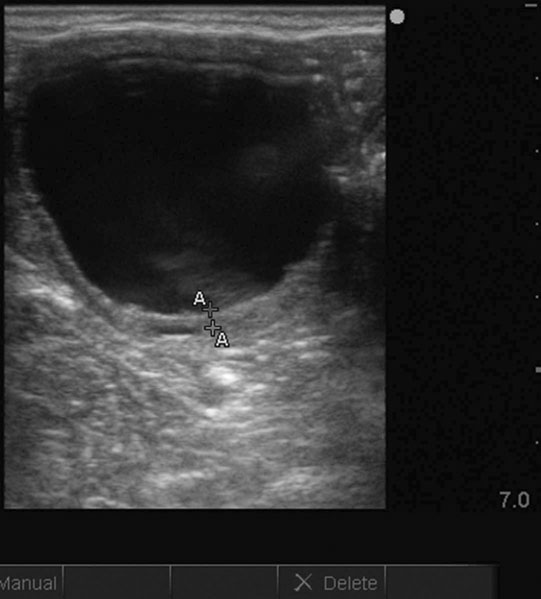
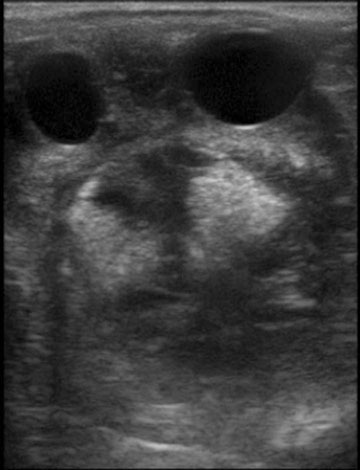
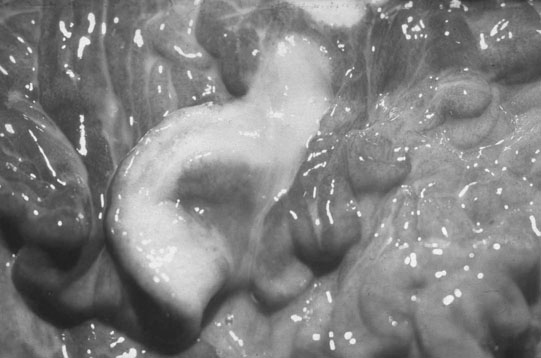
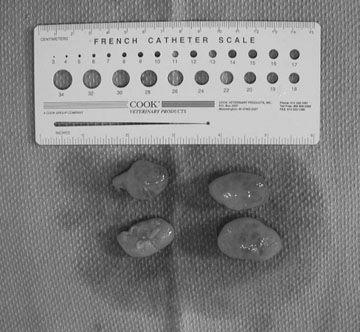
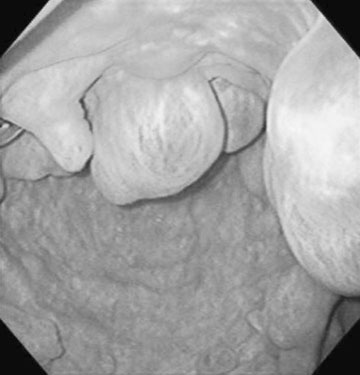
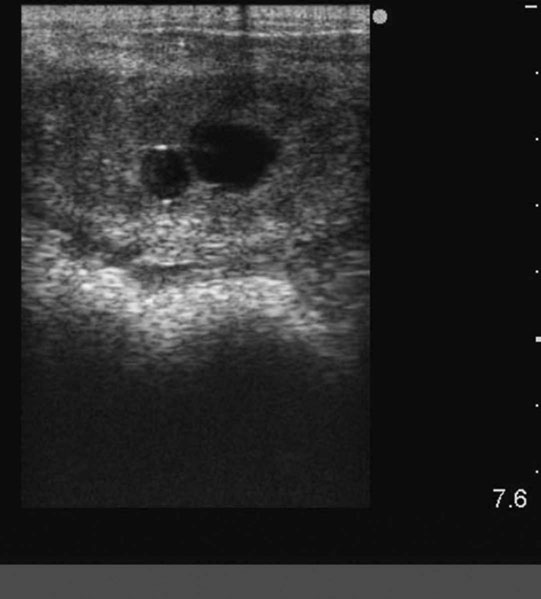
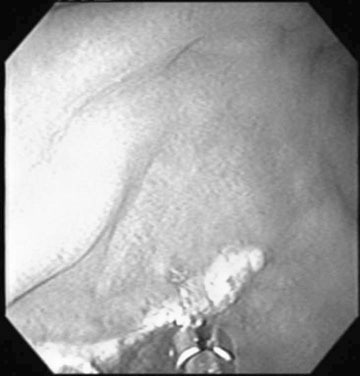
Endometrial cysts (Figs. 12.33–12.37)
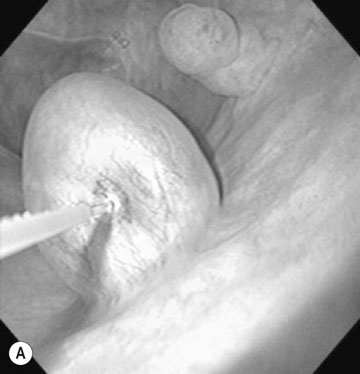
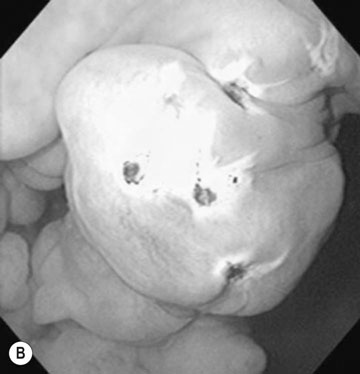
Diagnosis
Treatment
Persistent endometrial cups (Figs. 12.38–12.40)
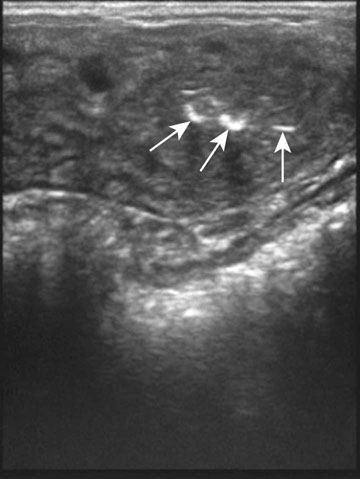
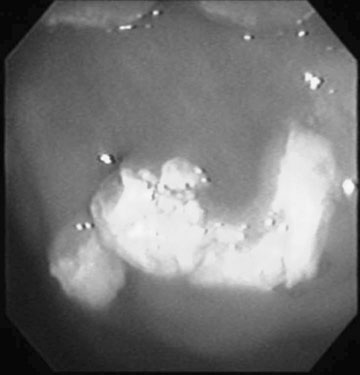
Diagnosis
Treatment
Non-infectious disorders (pregnant mares)
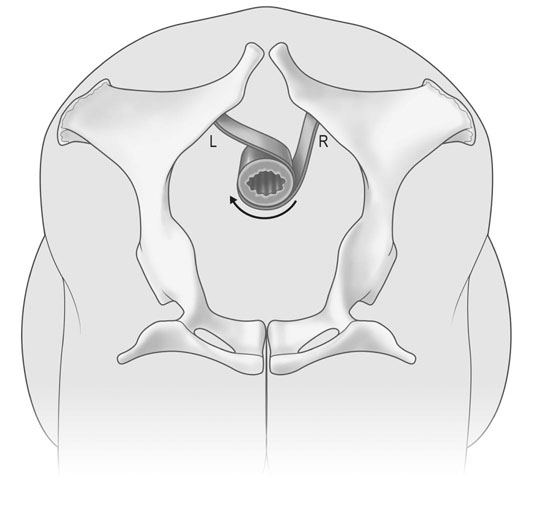
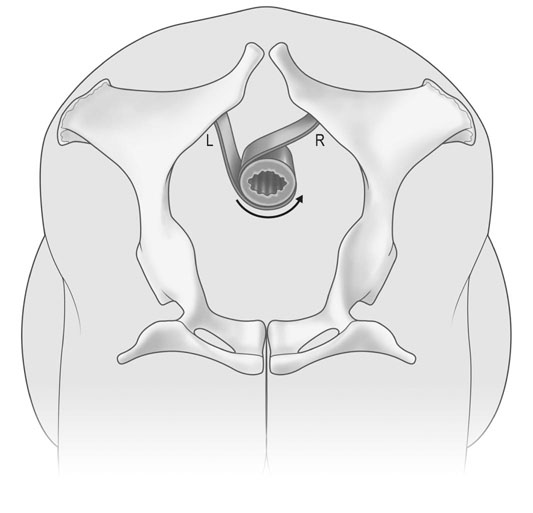
Uterine torsion (Figs. 12.41 & 12.42)
Treatment
Peripaturient hemorrhage (Figs. 12.43–12.46)
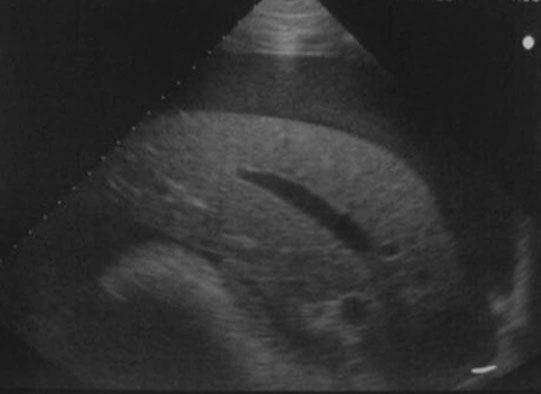

Diagnosis
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree