• The diagnosis may be difficult in some cases since liver enzymes and serum bilirubin are usually normal and any condition resulting in abnormal behavior and poor growth is a differential. Markedly elevated serum bile acids and blood ammonia with normal serum hepatic enzymes are highly supportive of the diagnosis. The diagnosis can be confirmed by a portogram or by nuclear scintigraphy. • Differential diagnosis: The main clinical signs are abnormal behavior and poor growth. Therefore any condition producing this combination of signs could be considered as a differential. This would include: brain abscesses, mesenteric/lung abscesses, microencephaly or hydrocephalus and chronic intestinal inflammation. Morgan foals with hyperammonemia can also have similar signs. • Surgical repair has been performed in one foal. Single extrahepatic shunts would have a better prognosis than multiple or intrahepatic shunts. • Ante-mortem diagnosis has not been made in any reported cases but hepatobiliary scintigraphy has been used in other species for diagnosis. • It may be clinically impossible to differentiate from acquired obstructions in biliary outflow. • Post-mortem of the affected foals revealed a large firm liver with abscence of the main bile duct with intrahepatic biliary hypertrophy. There are no treatment options. • The diagnosis is based upon signalment and clinical signs in addition to measurement of blood ammonia. • Blood ammonia levels are often very high (300–600 µmol/L). If blood ammonia is to be measured, it should be performed either ‘in house’ with a control sample or, if it is sent to a laboratory, serum should be immediately collected and frozen and a control sample handled in the identical way. • Although the disease has been uniformly fatal, some cases will improve for several days with supportive therapy only to have a second onset of severe neurological signs later. The onset of clinical signs attributable to liver disease is invariably acute, as the liver has a large functional reserve and normally greater than 75% of the liver mass must be functionally lost before clinical signs are apparent (see Table 2.1). Thus when signs of hepatic failure become obvious damage is usually severe, whether this is of chronic or acute onset. The organ does, however, have some regenerative capacity and may regain some, if not all, of its complex functions even after severe acute insults. Acute hepatic damage therefore provides the clinician with therapeutic opportunities while chronic severe changes are usually frustratingly unmanageable. Table 2.1 Clinical signs associated with hepatic insufficiency Hyperbilirubinemia may result from: 1) Increased production of bilirubin • Hemolysis (such as equine neonatal isoerythrolysis or equine babesosis) or intracorporeal hemorrhage (uterine artery hemorrhage) with subsequent reabsorption of heme from RBCs results in an elevation of unconjugated bilirubin, although occasionally there may be elevations in conjugated bilirubin as well, due to hepatic spillover. 2) Impaired hepatic uptake or conjugation of bilirubin result in increased levels of unconjugated bilirubin. • Acute or chronic hepatocellular disease. (Conjugated bilirubin may also increase in hepatocellular disease.) • Administration of certain drugs. Steroids inhibit bilirubin uptake in all species. Heparin may also impair bilirubin uptake. • Anorexia frequently causes icterus in horses and is believed to be related to reduced stores of lignadin, a hepatic enzyme responsible for extracting unconjugated bilirubin from albumin in the sinusoidal blood. • Premature and neonatal foals are more susceptible to retention icterus than adults and this is also believed to be related to lower stores of ligandin in neonates. 3) Impaired excretion of bilirubin through a blockage of bile flow results in regurgitation icterus with an increase in the conjugated bilirubin (>25%). This can be seen with: The establishment of the cause of icterus is obviously important and biochemical analysis of the proportions of conjugated and non-conjugated bilirubin in circulating blood may be required to accurately identify the type of icterus which is present and thereby provide indications as to its origin. This is a complex syndrome of abnormal mental status resulting from increased neuronal inhibition that accompanies severe hepatic insufficiency. There are no specific features of HE that allow it to be distinguished from other causes of cerebral dysfunction and a diagnois is usually made based on abnormalities of serum liver enzymes or the accompaniment of other clinical signs of liver disease. HE has been divided into four clinical stages (Table 2.2). Table 2.2 Stages of hepatoencephalopathy 1) Gastrointestinal-derived neurotoxins, e.g. ammonia • Ammonia has a toxic effect on the cell membrane of neurons by inhibiting Na+, K+-ATPase causing a depletion of adenosine triphosphate. • Hyperammonemia also causes disturbances in CNS energy production by altering the tricarboxylic acid cycle. • Detoxification of ammonia by brain astrocytes leads to accumulation of glutamate and glutamine. Glutamine accumulation in astrocytes then results in cell swelling and death. • Prolonged exposure of neuronal tissue to ammonia also results in downregulation of glutamate receptors resulting in decreased excitatory transmission. 2) False neurotransmitter accumulation following plasma amino acid imbalance • During liver failure false neurotransmitters such as octopamine and phenylethanolamine increase while true neurotransmitters such as norepinephrine and dopamine decrease with a net effect of reduced neuronal excitation and increased neuronal inhibition. 3) Augmented activity of γ-aminobutyric acid (GABA) in the brain • Binding of GABA to pre-synaptic neurons results in generation of an inhibitory post-synaptic potential. The GABA receptor has interactive binding sties for benzodiazepines and barbiturates. Improvement in clinical signs is seen when patients are treated with the benzodiazepine receptor antagonist flumazenil. Liver biopsy, which is a relatively safe and simple procedure, provides definitive histological evidence of the type and extent of the pathological process. As almost all the significant hepatic disorders occurring in the horse are diffuse, biopsy is a most useful aid to their diagnosis. Although chronic hepatic failure may be accompanied by blood clotting disorders the procedure is seldom accompanied by dangerous blood loss. Some of the more common means of assessment of liver pathology are shown in Table 2.3. Table 2.3 Some of the more commonly employed aids to the diagnosis of hepatic disease in the horse 1Bromosulphothalein Clearance/Retention Test. 2Lactate dehydrogenase isoenzyme-5 (also found in skeletal muscle). • Sedation. Horses with HE are often difficult to control and sedation is often required to allow for other treatment, avoid self-inflicted injury and provide safety for the nursing staff. Many tranquilizers are metabolized by the liver and should therefore be used cautiously. Xylazine or detomidine in small doses are safest. Diazepam should be avoided as it enhances the effect of GABA and may exacerbate signs. • Fluid therapy. Fluid deficits and acid–base imbalances should be addressed. Extreme caution should be taken if administering bicarbonate- or lactate-containing fluids as they may result in elevations of blood ammonia levels. Hypokalemia or alkalosis result in increased renal production of ammonia with a corresponding increase in diffusion of ammonia into the CNS, thus treatment with potassium or acidifying fluids may be necessary. As many affected horses are anoretic, continuous infusion of dextrose 5% at a rate of 2 ml/kg/h may be beneficial. • Reducing toxic metabolites. Reducing the production or decreasing the absorption of toxic protein metabolites is also an important part of therapy. Methods to reduce production include the oral administration of antibiotics (e.g. neomycin) or lactulose (a syrup containing lactose and other disaccharides). A disadvantage of both is that they may produce diarrhea. Lactulose is also expensive for long-term therapy. Other means of reducing production include alteration of intestinal flora by administration of pro-biotics such as Lactobacillus acidophilus. Administration of a low-protein diet (see below) is also advised although this has not consistently reduced signs of HE in humans. Zinc is an important co-factor in many urea cycle enzymes and supplementation may be warranted. Decreasing absorption of toxic metabolites is achieved through the administration of mineral oil or magnesium sulfate. • Dietary management. Once horses with hepatic insufficiency become appetent they can be best managed by dietary control. Diets high in carbohydrates, low in protein and rich in BCAAs (branch chain amino acids) should be fed. Oat or other types of grass hay are best. Alfalfa and legumes should be avoided. Grazing should be encouraged. Small feeds several times a day are best because of impaired gluconeogenesis. Vitamin B1, K1 and folic acid should be administered weekly. • Other treatments. Anti-inflammatory drugs may be beneficial and include flunixin meglumine, dimethyl sulfoxide and pentoxifylline. Other experimental drugs have been used in human and small animal medicine and may have a future role for management of hepatic insufficiency in horses.
Conditions of the liver, spleen and pancreas
Developmental disorders
Hepatic disorders
Portosystemic shunt
Diagnosis and treatment
Biliary atresia
Diagnosis
Hyperammonemia of Morgan foals
Diagnosis and treatment
Hepatic insufficiency
Common
Less common
Infrequently reported
Icterus
Photosensitization
Acites
Hepatic encephalopathy
Diarrhea
Dependent abdominal edema
Depression
Bilateral laryngeal paralysis
Steatorrhea
Anorexia
Hemhorragic diathesis
Tenesmus
Colic
Generalized seborrhea
Weight loss
Pruritis
Endotoxic shock
Polydipsia
Hemolysis
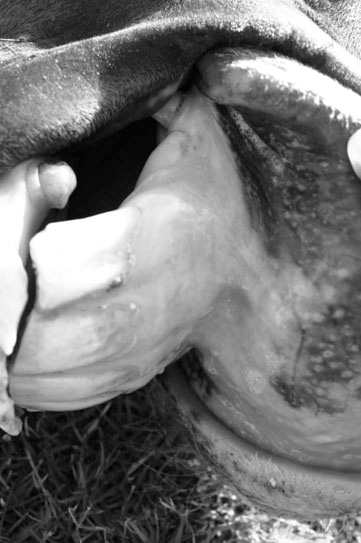
Icterus (Figs. 2.1 & 2.2)
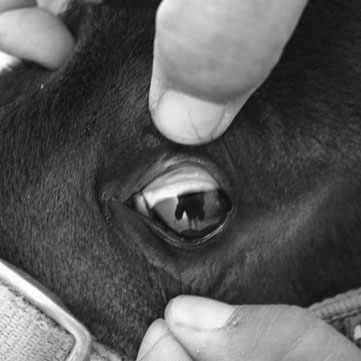
Hepatic encephalopathy (HE) (Figs. 2.3–2.6)
Stage
Mental status
Clinical signs/mental status
I
Mild confusion. Decreased attention and irritability
This stage is often missed as the signs are very subtle
II
Drowsiness, lethargy, inappropriate behavior and disorientation
Head-pressing, circling, aimless walking, mild ataxia, yawning and lethargy
III
Somnolent but rousable, marked confusion, amnesia, aggressive uncontrolled behavior
Somnolence with either minimal or hyperresponsiveness to external stimuli. Aggressive or violent behavior
IV
Coma
Coma +/– seizures

Differential diagnosis of abnormal behavior patterns: neurological disorders.
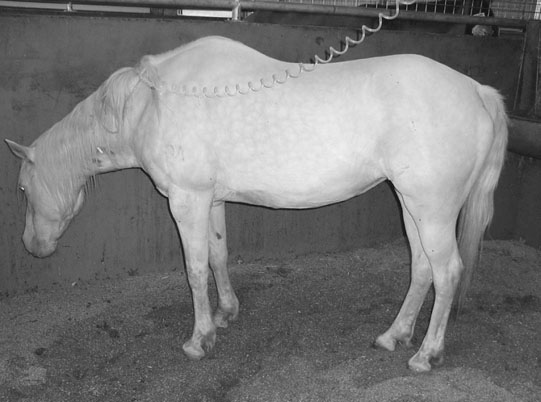
Polydipsia, polyuria and the hepatorenal system
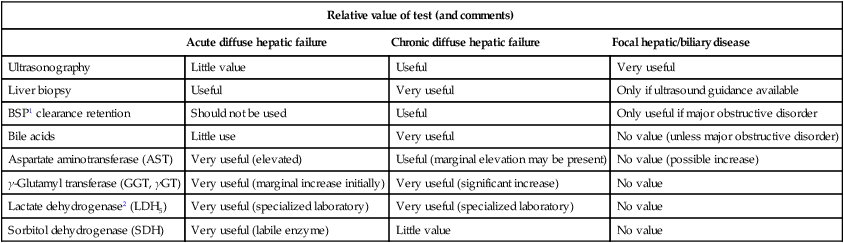
Diagnosis of liver disease
Relative value of test (and comments)
Acute diffuse hepatic failure
Chronic diffuse hepatic failure
Focal hepatic/biliary disease
Ultrasonography
Little value
Useful
Very useful
Liver biopsy
Useful
Very useful
Only if ultrasound guidance available
BSP1 clearance retention
Should not be used
Useful
Only useful if major obstructive disorder
Bile acids
Little use
Very useful
No value (unless major obstructive disorder)
Aspartate aminotransferase (AST)
Very useful (elevated)
Useful (marginal elevation may be present)
No value (possible increase)
γ-Glutamyl transferase (GGT, γGT)
Very useful (marginal increase initially)
Very useful (significant increase)
No value
Lactate dehydrogenase2 (LDH5)
Very useful (specialized laboratory)
Very useful (specialized laboratory)
No value
Sorbitol dehydrogenase (SDH)
Very useful (labile enzyme)
Little value
No value
Treatment of hepatic insufficiency
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree