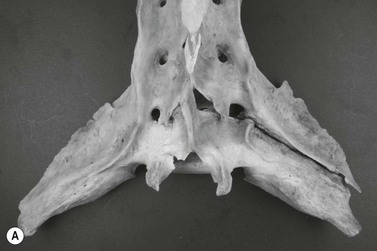
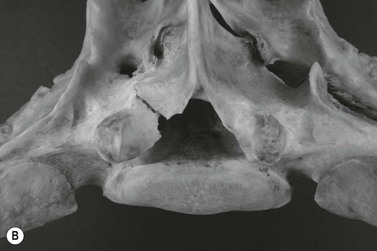
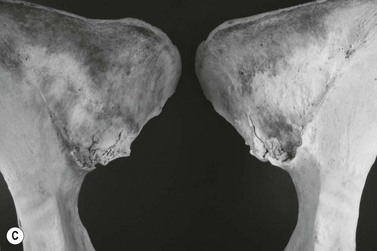
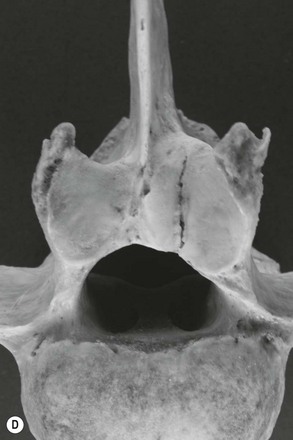
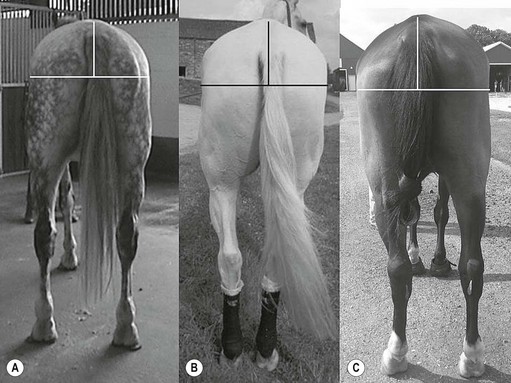
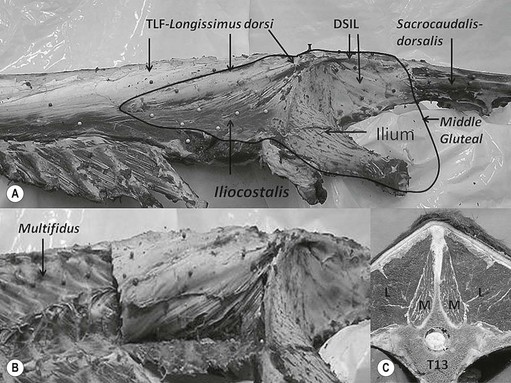
Chapter 17 Equine sport sciences are evolving along similar lines to human sports sciences. There has been considerable research on normal and pathological gaits, and the high prevalence of locomotor injuries in athletic horses is well established (Ely et al., 2009; Scott, 2008). Physical therapy and rehabilitation play an important role in performance enhancement, injury prevention and restoration of full function during recovery from injury. This is particularly relevant in relation to back pain and lameness prevention strategies in horses participating in exercise or performance-related activities, including racing, and in horses stabled in smaller operations for pleasure activities, as there is increased risk of lameness for active horses (n = 3925 horses in 138 randomly selected yards; Ross et al., 1998). As described in previous chapters, numerous studies have described factors that may predispose the horse to injury, lameness and lack of or loss of performance (Burns et al., 2006). These include: morphology; conformation; training; environmental conditions; type of competition; age and sex; performance limitations; exercise effects on the neuromuscular and skeletal systems; and interaction between rider and horse. Rehabilitation encompasses broad-based concepts that relate to all the aforementioned factors with a focus on tissue healing, biomechanics and neuromotor control, which all ultimately affect equine locomotion. Veterinary sports medicine and rehabilitation follows in the footsteps of the human counterparts encompassing many professional fields and areas of research, including: physiotherapy (PT), osteopathy, chiropractic, and other complementary medicines. Objective measures of locomotor kinematics and kinetics (see Chapter 2) are useful in monitoring progress and obtaining outcome measures in rehabilitation, both clinically and for research purposes. Conventional diagnostic equipment can also be used in rehabilitation including electromyography (EMG) (Cottriall et al., 2008) and ultrasonography (McGowan et al., 2007b; Stubbs et al., 2010, 2011), along with relatively inexpensive functional measurement tools such as pressure algometry (Haussler& Erb, 2003, 2006a,b; Haussler, 2006; Varcoe-Cocks et al., 2006; Sullivan et al., 2008; De Heus et al., 2010), goniometry (Liljebrink & Bergh, 2010), digital photography, videography and the tape measure. However, as shown in human studies many of the measurement tools such as goniometry, used to document limb passive motion at the fetlock, carpus and hock are only reliable when used by the same investigator, with further studies needed to validate this tool in the horse (Liljebrink & Bergh, 2010). Complementary objective and subjective clinical outcome measures of performance and function are also vital in the rehabilitation setting which may include force plates to measure and monitor neurological signs and or lameness during the rehabilitation process (Clayton et al., 2003; Ishihara et al., 2009) and motion analysis to measure intervertebral and limb motion as described elsewhere in the text. For example, Faber and colleagues presented a single case study in a dressage horse with scoliosis at T10–L1 that caused severe loss of performance. After two sessions of thoracolumbar (TL) manipulation the effects were assessed using a validated kinematic analysis protocol (Faber et al., 1999, 2001) to measure TL motion and spatiotemporal parameters with the horse walking and trotting at standardized speeds on a treadmill. Spinal motion was quantified by range of motion in the three planes of motion and intervertebral pattern symmetry (Faber et al., 2000). Data were collected pre-treatment, 48 h after treatment and at weeks 3, 7 and 36. Treatment involved ‘direct short-lever thrust towards the neutral position of the areas where an impaired mobility had been noted during the clinical examination’, labeled as ‘orthomanual realignment technique’. Progressive mobilization exercises were also given (walking along serpentines). The aim of this review is to summarize equine rehabilitation from an evidence-based perspective utilizing references from databases, such as Pubmed. There is a long history of physical therapy in the treatment of animals. A book on physiotherapy for horses, written by the physiotherapist Sir Charles Strong, was published in 1967 in the UK. Two other early publications on physiotherapy for horses appeared in the 1970s (Hopes, 1970; Downer, 1978) focusing on back pain. The word rehabilitation comes from the Latin ‘rehabilitare’ meaning to make fit again. Traditionally this encompasses treatments designed to facilitate the process of recovery and restoration to a former capacity following an illness or injury. These are the founding principles of the physiotherapy profession as described by the World Confederation for Physical Therapy. Complementary medicine including PT in all species is concerned with identifying and maximizing quality of movement potential within the spheres of promotion of health, prevention of injuries, treatment/intervention, rehabilitation and, more recently, performance enhancement and sports medicine. Physiotherapy has been defined as ‘A holistic approach to the prevention, diagnosis and therapeutic management of pain, disorders of movement or optimization of function to enhance the health and welfare of the community from an individual or population perspective’. McGowan et al. (2007c) emphasized that one of the fundamental differences between PT and the medical or veterinary profession is that physiotherapists are trained to focus on the assessment and management of a patient’s function, rather than focusing purely on the specific patho-anatomical diagnosis. The aim of PT and rehabilitation is to restore function and promote tissue healing by assisting normal physiological processes through the application of manual therapy techniques, electromodalities and exercise-based regimens. Buchner and Schildboeck (2006) and McGowan et al. (2007c) recognized these specific areas: manual/manipulative therapies (PT, massage, chiropractic, osteopathy) and complementary alternative medicine (CAM) also known as physical or technical/modality based therapies (physiotechnical therapies): acupuncture, electrotherapy, exercise, hydrotherapy, laser therapy, magnetic field therapy, thermotherapy and therapeutic ultrasound. Rehabilitation in veterinary medicine involves the veterinarian as primary patho-anatomical diagnostician, a thorough objective functional assessment of the patient and consultation with other health professionals (McGowan et al., 2007a). Using knowledge and skills unique to these professions the patient’s movement potential is assessed, with all information and confounding factors being incorporated to establish an accurate functional diagnosis, problem list, management plan and goals. Clinically, and particularly in relation to research, it is imperative that during the rehabilitation process valid and reliable objective measurements are taken to determine accurate outcome measures. The integration of research into evidence-based practice continues to be a challenge in veterinary medicine and rehabilitation is no exception. Currently there is limited research in equine locomotion in relation to rehabilitation, though many animal models have been used for human rehabilitation research with the findings being integrated into current concepts of equine rehabilitation. Textbooks and review articles in the area of veterinary PT and rehabilitation include Animal physiotherapy: assessment, treatment and rehabilitation of animals (McGowan et al., 2007a). This is an evidence-based textbook that reviews the literature in relation to animal PT and is a useful reference with respect to outlining the foundations of rehabilitation and equine locomotion. A recent review Equine Physiotherapy: the science behind the profession (McGowan et al., 2007c) also highlights the potential input of PT in equine rehabilitation. Another useful text is Physical Therapy and Massage for the Horse which complements both evidence based and clinical practice (Denoix & Pailloux, 2005). In addition there are clinical and evidence based articles, chapters and textbooks (Porter, 2005; Bromiley, 2007; Henson et al., 2009; Pusey et al., 2010). The profile of rehabilitation and performance enhancement has also been heightened in recent years by the official use of physiotherapists during international equestrian competitions. In this context, equestrian sports are rapidly catching up to other international competitive sports, such as football and athletics, in which individuals and teams utilize the professional service of physiotherapists not only in treatment of injuries, but also in maintenance and enhancement of performance. In addition, there is a growing body of literature describing research studies in equine rehabilitation (e.g. Wakeling et al., 2006; Haussler et al., 2007, 2010; Ramon et al., 2004; Wennerstrand et al., 2006; Xie, 2005; Clayton et al., 2008, 2010a,b, 2011a,b; De Heus et al., 2010; Stubbs et al., 2010, 2011). Many forms of PT, manual therapies and alternative medicine can play a role in the horse’s return to optimal performance. Human therapeutic texts should be consulted to further the reader’s understanding of manual therapies, soft tissue mobilization/massage (Boyling et al., 2004), myofascial pain and dysfunction techniques (Simons et al., 1999), electrotherapy (Watson, 2008) and integrative therapeutic approaches relating to mechanical passive constraints of locomotion and neuromotor control including facilitation and strengthening techniques (McGill, 2007; Mooney, 2007; Lee & Vleeming, 2007). The clinical need for rehabilitation interventions and justification for research into this field are verified by the amount of wastage in equine sports due to musculoskeletal injuries (Peloso et al., 1994; Valentine, 2008; Barr et al., 2009; Ely et al., 2009). Statistics related to longevity and life spans are often collated according to insurance company records. These indicate that diseases of the musculoskeletal system are the predominant cause of death in sport horses (Clausen et al., 1990; AGRIA, 1995 cited in Wallin et al., 2000; Heisele, 1995), whereas diseases of the digestive system are the most frequently reported cause of death in companion horses (Baker & Ellis, 1981). In a follow-up 5-year survival study of 2495 horses with at least one costly veterinary-care event and 15 576 horses with no costly veterinary care event evaluated for 1 year, the risk of death increased linearly with age and with increasing life-insurance value (Egenvall et al., 2006). Horses with previous lameness had the lowest survival. Horses with previous locomotor problems continued to have considerably more veterinary-care events and higher costs for locomotor problems during the follow-up period (Egenvall et al., 2008), highlighting the potential need for further and improved rehabilitation strategies. The most common disease in 107 310 horses of varying age, gender, breed and use requiring veterinary care covered by complete insurance in Sweden from 1997 to 2000 was arthritis, which most frequently affected the fetlock (28%) or multiple joints (16%) (Penell et al., 2005). In a 5-year study of 5140 horses from 136 riding schools the overall yearly incidence rate was 1584 events of veterinary care per 10 000 horse-years at risk (Egenvall et al., 2009). The total and diagnostic mortalities were 790 and 763 deaths per 10 000 horse-years at risk. Rates varied substantially among riding schools. For locomotor problems the rates were 1116 events of veterinary care and 524 deaths per 10 000 horse-years at risk. For the outcome veterinary care for locomotor problems, the hazard ratio (HR) increased with increasing life-insurance value, was higher in horses than ponies, and was higher in Warmbloods than other horses. The HR increased by 33% for each year of age at entry. Age at entry ≥8 years was associated with decreased HR due to locomotor problems. Wallin et al. (2000) investigated the longevity, causes of death and culling of Swedish Warmblooded and Coldblooded horses via a retrospective owner questionnaire. Data were retrieved from horses born 1968–1986 participating in Riding Horse Quality Tests as 4-year-olds, with information about the horses available until 1990. Of the 1847 Warmbloods, 503 were dead, 85% had competed in different sporting disciplines and many were used as leisure horses after retirement from competition. Data also included horses of the Swedish Cavalry Horse Foundation born between 1970 and 1975 with information available until 1989: 208/344 Warmbloods and 97/204 Coldbloods were dead. The most common causes of death were musculoskeletal diseases (56–57%), respiratory diseases (8–9%), diseases of the digestive system (5–6%), accidents (3–9%), and causes unknown in 13.0% of Warmblooded horses. In the Coldblooded horses death was attributable to temperamental disorders (23%), diseases of the musculoskeletal system (14%) and hoof diseases (8%). A written questionnaire among (Swiss) horse owners (n = 2912 horses and ponies) indicated that a veterinarian examined 718 horses (24.7% of the sample population) within the 12 months prior to the survey (Knubben et al., 2008). Orthopedic and traumatic disorders (41.5%) had the largest proportion, followed by gastrointestinal (27.1%) and respiratory (14.0%) diseases. Half of the lameness cases occurred as a direct consequence of an injury. In 25.6% of all cases diagnosed by a veterinarian, alternative therapeutic methods were used either in addition to traditional medicine or exclusively (Knubben et al., 2008). A UK study surveyed owners of registered dressage horses (n = 2554) reporting that 33% of horses had been lame at some time during their career, with 24% of these within the previous 2 years (Murray et al., 2010). A number of factors were associated with the occurrence of lameness in the last 2 years, with increased risk for older and bigger horses; use of arenas that were indoors, not privately owned, and that became deeper in wet conditions or were sand-based; use of and longer time spent in horse-walkers (cause or effect); not lunging; shorter turnout time; and back pain. It is well established in the literature that flexor tendon and suspensory ligament injuries are the most frequently reported injuries in sports requiring the horse to perform at high speed and over jumps (Rooney & Genovese, 1981; Jeffcott et al., 1982; Rossdale et al., 1985; Mohammed et al., 1991; Marr et al., 1993; Colbourn & Yovich, 1994; Reef, 1998). In the Thoroughbred (TB) racing industry musculoskeletal injuries account for three times more wastage than all other medical problems (Rossdale et al., 1985; Robinson & Gordon, 1988). More than half of the 2–4-year-old racehorses become lame and 20% of all racehorses eventually suffer a career ending musculoskeletal injury or disease (Bourke, 1995). Ely et al. (2009) found that fractures, tendon and suspensory ligament injuries were important causes of morbidity and mortality in 1223 National Hunt racehorses in training in the UK. Ramzan and Palmer (2010) recently investigated musculoskeletal injuries in three training yards (616 horses) in Newmarket (UK) finding a total of 248 injuries that occurred in 217 horses with fractures of the tibia (20.7%) and proximal phalanx (14.5%) being the most common. A post mortem study of the third metacarpal and metatarsal bones of 64 TB racehorses reported a 67% prevalence of palmar/plantar osteochondral disease (Barr et al., 2009), which is considered to be a consequence of repetitive cyclical high intensive overload resulting in arthrosis (Pool, 1996). This painful condition is recognized in most racing breeds including TB (Arthur et al., 2003; Pilsworth, 2003), Standardbreds (Mitchell et al., 2003; Torre, 2003), Quarter Horses (Lewis, 2003) and Scandinavian Coldblooded trotters (Ertola & Houttue, 2003). Overall, the incidence of catastrophic musculoskeletal injury of Thoroughbreds in the USA has been reported as 1.2/1000 race starts (Hernandez et al., 2001). Incidence of injury was significantly higher for turf races (2.3/1000 starts) than for dirt races (0.9/1000 starts). The number of days since the last race (≥33 days) was associated with a higher risk of injury. A study of 265 Danish Standardbreds evaluated over a 5-month period showed that a change of trainer affected the risk of lameness (Vigre et al., 2002). Compared to the period in which horses had been with the same trainer for >3 months, horses that entered a different training regime within the past 1.5–2.5 months had a higher risk of lameness. Participation in races increased the risk of lameness significantly in the 5 days following a race. In a (USA) study of 357 lameness cases, 78.6% were reported to have recovered after a median duration of 18 days (Ross et al., 1999). Some type of treatment was administered in 82.9% of lameness incidents. Of 619 total treatments used, 53.2% were administered or applied by a veterinarian. Horses experiencing hoof lameness were more likely to recover than those with other types of lameness. Horses that had participated in exercise-related activities during the study period and prior to the development of lameness were more likely to recover. Treatment of the lameness was associated with an increased likelihood of recovery. Cases with a veterinarian involved in the diagnosis were associated with a decreased likelihood of recovery and a longer duration of lameness, which might indicate that these cases were more complex or severe. Although cases treated for lameness were more likely to recover, treatment did not affect lameness duration (Ross et al., 1999). Gibson et al. (2002) reported the frequency of soft tissue injury in 70 elite sport horses competing at the 2000 Sydney Olympics in dressage (8), show jumping (15), and eventing (47). As reported previously (Reef, 1998), suspensory desmitis (n = 4) was most common in dressage horses affecting both fore and hind limbs. Some were able to compete despite having suspensory desmitis, others had to withdraw. The most common lesion in show jumpers (n = 10) was suspensory desmitis including desmitis of the extensor branches, with four medalists amongst this group. The authors suggest that show jumpers can continue to compete successfully with good management despite chronic low-grade injury. Tendon and ligament injuries occurred more frequently in eventers due to the requirement for speed and jumping. Soft tissue lesions were present in 45/47 horses that were presented to the veterinary clinic, 10 horses had multiple affected sites, most commonly superficial digital flexor tendonitis and/or suspensory desmitis (proximal or branches), tenosynovitis, desmitis, core lesions and diffuse loss of echogenicity. Many did not complete due to retiring on course (n = 10), elimination on course (n = 3) or withdrawal before the third inspection (n = 6). However, 20 horses passed the veterinary inspections and completed the event, whilst adhering to the veterinary rules of the Fédération Equestre Internationale (FEI) (2011) with respect to non-pharmaceutical treatments (www.fei.org). Fourteen horses were presented for veterinary evaluation before the start of the competition, only one of these completed all three phases (proximal suspensory desmitis), five did not complete because of the existing injury at the time of arrival in Sydney or during training leading up to the Games. The remaining eight horses fell or were eliminated during competition for reasons other than a lesion. A study in event horses training to compete in Concours Complète Internationale (CCI) showed that 21% did not start due to injury: 43% had soft tissue injuries, 33.3% involving the superficial digital flexor tendon and 30.6% involving the suspensory ligament (Singer et al., 2008). Of those competing in the CCI, the most common injuries included lacerations and abrasions to the carpus and stifle, superficial digital flexor tendonitis and exertional rhabdomyolysis. These injuries were significantly higher in CCI competitions than at one-day events, most likely due to the increase in demands of athletic performance. Scott (2008) performed an extensive literature review summarizing the musculoskeletal injuries in non-racing Quarter Horses which included palmar foot pain, osteoarthritis of the proximal interphalangeal joints, pastern fractures, suspensory ligament desmitis, osteoarthritis of the distal tarsal joints, stifle injuries and back pain. The review presents the current literature on the diagnosis, treatment and rehabilitation strategies including concepts of injury predisposition and prevention. A retrospective study by Dabareiner et al. (2005a) identified types of musculoskeletal problems associated with lameness or poor performance in 118 horses used for barrel racing. The forelimbs were more frequently affected than the hind limbs, with forelimb foot pain and osteoarthritis of the distal tarsal joints being the most common abnormalities. Reasons for presentation were lameness (61%) or poor performance (39%). The most common performance change (41%) was refusal or failure to turn properly around the first barrel. Dabareiner et al. (2005b) also reported on 118 team roping horses presenting with lameness or poor performance. A significantly greater proportion (74/118) of horses used for ‘heading’ (roping the steer around the horns) presented for examination compared to (44/118) horses used for ‘heeling’ (roping around the hind limbs of the steer). Most horses examined for poor performance were lame, however, the biomechanical loading patterns influenced the type of lameness: ‘headers’ had more right forelimb lameness (35%) compared with ‘heelers’ (16%); ‘headers’ had a significantly greater proportion of bilateral forelimb lameness (24%) compared with ‘heelers’ (9%), but ‘heelers’ had more bilateral hind limb lameness (7%) compared to ‘headers’ (0%). The most common musculoskeletal problems for ‘headers’ were pain limited to the distal sesamoid (navicular) area, with or without osteoarthritis of the distal tarsal joints and soft tissue injury in the forelimb proximal phalangeal (pastern) region. The most common signs of pain in ‘heelers’ were in the navicular area, osteoarthritis of the metatarsophalangeal joints and osteoarthritis of the distal tarsal joints. Veterinarians are more often consulted for the more complex lameness cases, with 25% of the cases diagnosed by a veterinarian being reported to receive alternative therapeutic methods (Knubben et al., 2008). Physiotherapists who work in racehorse training yards routinely treat horses’ backs and hindquarters. It has been reported that racehorses presented for PT showing pelvic bony asymmetry, muscle atrophy of the hindquarters and/or spasm or tenderness on palpation of the gluteal muscles should alert the physiotherapist to the possibility of impending pelvic or hind limb fracture (Hesse & Verheyen, 2010). Equine back pain is a condition where PT and rehabilitation strategies are clinically useful and is an area of expanding research interest. As discussed in Chapter 10, back problems are associated with alterations of gait and performance (cause and/or effect). Improved diagnostic capabilities combined with increased clinical and research interest has raised awareness of the importance of equine back pain. Historically, Jeffcott (1980) reported the prevalence of equine back pain to be 0.9% in general veterinary practice, 2% in TB racehorse practice, 5% in a veterinary school referral practice, 13% in a mixed equine practice (dressage, show jumpers, eventing), and 47% in a spinal research clinic. These figures can be compared with a reported rate of 94% at an equine chiropractic clinic (Haussler, 1999a, 2000). Racehorse trainers in Sydney, Australia reported back problems to be in the top quartile ranked conditions (Bailey et al., 1997), and one of the most common injuries preventing training and racing. In a survey of registered dressage horse owners in the UK, 25% (644/2554) of the respondents indicated that their horse had a ‘back problem’ (Murray et al., 2010), though the majority (80%) had not been diagnosed by a veterinary surgeon. Of those that sought treatment (447/644) complementary therapy was the most common (63%) followed by saddle fit (24%); veterinary involvement (20%); rest (20%); a change in training (13%); and other (6%) (Murray et al., 2010). From the 25% of horses thought to have back pain, 2.5% received veterinary care alone and 3% received a combination of veterinary care and complimentary therapy, highlighting the need for a team approach to treatment and owner education. Interestingly horses reported to have a ‘previous back problem’ that was resolved by complimentary therapy or rest were more likely to have been lame in the last year. Historically Jeffcott (1979) reported that in 190 horses treated for chronic back problems 57% recovered completely, 17% showed no improvement and 38% had a recurrence or continuation of signs of low-grade back pain. This highlights the need for research into rehabilitation strategies and long-term management of chronic and recurrent back pain in horses. Equine back pain often presents with more than one lesion or problem area including limb lameness. Osseous lesions of the thoracolumbar (TL) spine and the lumbopelvic complex are widely recognized as significant causes of equine back pain, poor performance, loss of performance, and altered back and limb kinematics (Jeffcott, 1975, 1980; Townsend et al., 1986; Denoix, 2005, 2007; Wennerstrand et al., 2004; Cousty et al., 2010; van Weeren et al., 2010). Such lesions may arise as primary injuries to the vertebral column and related structures, or secondary to other musculoskeletal injuries (Landman et al., 2004; Meehan et al., 2009; Girodroux et al., 2009). In a population of 805 horses (70% dressage, 20% show jumpers, 10% trotters) with orthopedic problems, 74% that were presented with a back problem were lame and 32% of those presented for lameness had a back problem (Landman et al., 2004). These percentages were significantly higher than those recorded in the same study for a control population of 399 horses, in which 20% were lame and 12% had back problems. Therefore there is a strong association between lameness and back problems. Even subtle hind limb lameness can cause changes in spinal kinematics at trot involving increased range of motion and hyperextension in the TL spine and reduced range of motion in the lumbosacral region (Gomez Alvarez et al., 2007a). It is widely reported that the TL spine is predisposed to damage and/or pain (Wennerstrand et al., 2004). A retrospective study by Jeffcott (1980) reviewed 443 cases of equine back pain. The primary pathological lesions associated with TL pain were vertebral lesions (38.6%), soft tissue injuries (25%), sacroiliac strain (13%) and non-TL lesions (13%). Vertebral lesions were predominantly crowding and over-riding dorsal spinous processes (DSP), which were most common beneath the saddle from T12–T17 and were most prevalent in young adult to middle aged horses used for jumping or dressage and in Thoroughbreds (TB) with short backs. Soft tissue lesions were predominantly in the longissimus dorsi muscles and supraspinous ligament in the caudal withers and cranial lumbar regions. Specific causes of back pain that have now been identified include muscle strain (Jeffcott & Dalin, 1980; Piercy & Weller, 2009); ligamentous lesions (Jeffcott, 1980; Henson et al., 2007; Tomlinson et al., 2003); fractures of the TL and/or lumbo-pelvic complex (Sumner, 1948; Mason, 1971: Jeffcott & Whitwell 1976; Vaughan & Mason 1976; Haussler & Stover, 1998; Driver & Pilsworth, 2009); vertebral body osteophytes and spondylosis (Geres, 1978; Jeffcott, 1980; Haussler et al., 1999b; Meehan et al., 2009); osteoarthritis and ankylosis of the inter-transverse and or lateral inter-transverse joints (Mitchell, 1933; Stecher & Goss, 1961; Smythe, 1962; Haussler et al., 1999b); impingement of the DSPs (Roberts, 1968; Jeffcott & Hickman, 1975; von Salis & Huskamp, 1978; Walmsley et al., 2002; Cousty et al., 2010); sacroiliac disease (Rooney, 1977; Jeffcott, 1980; Jeffcott et al., 1985; Haussler et al., 1999b; Goff et al., 2008); degenerative intervertebral disc disease (Hansen, 1959; Rooney, 1970; Taylor et al., 1977; Townsend et al., 1986; Denoix, 2007); and osteoarthritis of the synovial intervertebral articulations (facet joints) (Jeffcott, 1980; Haussler et al., 1999b; Girodroux et al., 2009; Cousty et al., 2010; Stubbs et al., 2010). Historically, the most widely documented osseous lesion is impingement or over-riding of the DSPs (Jeffcott, 1980; Walmsley et al., 2002; Erichsen et al., 2004; Cousty et al., 2010). More recently, osteoarthritis of the facet joints has been identified as a source of pain and dysfunction (Denoix & Dyson, 2003; Girodroux et al., 2009; Stubbs et al., 2010). Nuclear scintigraphy showed moderate to intense increased radiopharmaceutical uptake in the facet joints of horses with pain in the region from T13–L1 compared with clinically normal horses (Gillen et al., 2009). However, only 61.5% of 67 horses with back pain associated with radiographic evidence of osteoarthritis had increased radiopharmaceutical uptake in one or more facet joints, highlighting the inherent difficulties, even with advanced technology, in localizing the cause of back pain and loss of function. Haussler et al. (1999b) highlighted the potential under-diagnosis of TL vertebral or pelvic lesions in a post mortem study of 36 TB racehorses that were euthanized for reasons unrelated to back pain. An alarming rate of osseous lesions was reported in the caudal thoracic and lumbar regions. Degenerative changes were observed at lumbar intertransverse joints and sacroiliac articulations in all specimens, with variable degrees of degenerative changes of the TL articular processes in 97% of specimens. Impingement of the DSPs (92%) and transverse processes (97%) was very prevalent, with many specimens having widespread and severe osseous changes including stress fractures of the facet joints. The relationship between the changes observed at necropsy examination and the presence of pain or loss of function could not be established. Stubbs et al. (2010) reported osseous pathologies in a group of 22 TB racehorses euthanized at the Hong Kong Jockey Club for reasons unrelated to back pain. Osseous lesions were graded as mild, moderate or severe at vertebral levels from T9–Ca1 in a non-cumulative fashion. All horses exhibited lesions of moderate severity and 77% had evidence of severe (grade 3) osseous pathology at various sites in the TL spine and pelvis (Fig. 17.1). Turner (2003) evaluated 5352 medical records from 1997 to 2002 at a veterinary teaching hospital and found the occurrence of true back problems to be 2.2% in the lameness caseload of mixed breeds and sporting disciplines. One hundred and twenty-four horses presented with the complaint of back pain with the diagnosis confirmed in 102 (82%) cases. An additional 16 horses that were presented for other problems were diagnosed with back problems. The most prevalent lesions involved the sacroiliac area (66). Other problems included kissing spinous processes, dorsal spinous ligament injuries, muscle pain, wither injuries, polysaccharide storage myopathy (PSSM) and saddle fit problems. Sixteen horses also had limb lameness. The treatment protocol included administration of systemic anti-inflammatories and/or a local anti-inflammatory injection and a variable combination of acupuncture, chiropractic techniques, massage therapy, electro-stimulation, magnetic therapy, therapeutic ultrasound, extracorporeal shockwave and training (exercise) management. Follow-up information was available on 112 horses with 90% returning to work; however, 15 of those horses did not return to their previous level and were retired or used for other activities. Of the 86 horses that returned to their previous level of work, 60% did not need further therapy but the remaining 40% continued to receive some form of therapy. The need for therapy was based on the owner’s and trainer’s impression of the horse’s behavior. The rate of successful return to previous level of work is much higher than reported by Jeffcott (1979), which may reflect advancements in veterinary diagnostics, medical management and the implementation of complementary therapies as part of the rehabilitation strategy. There is a smaller body of literature describing the equine cervical spine, neck pain and pathology. Studies of cervical osseous pathologies have focused mainly on cervical vertebral compressive myelopathy (van Biervliet et al., 2006; Levine et al., 2007). Arthropathy of the cervical facet joints has been cited in the aetiology of reduced performance in the horse and has been reported to cause forelimb lameness (Ricardi & Dyson, 1993), stiffness in movement, neck muscle atrophy, and neck pain (Beck et al., 2002; Dyson, 2003). A recent examination of six cadaveric necks by Claridge et al. (2010) developed a three-dimensional model of the cervical facet joints based on radiographs and computed tomography (CT) images and used the model to determine that effusion within the articular facet joints of the cervical spine is unlikely to cause compression of the spinal cord, which is known to be associated with neurological manifestations (Ricardi & Dyson, 1993). In vitro kinematic studies of the cervical spine have demonstrated that dorsoventral flexion and extension, axial rotation and lateral bending take place at each of the intervertebral joints with the largest ranges of motion in the upper (occiput to C2) and lower (C5–T1) joints (Clayton & Townsend, 1989a). There is a general reduction in overall cervical spinal mobility from foal to adulthood (Clayton & Townsend, 1989b). In vivo kinematics of the cervical spine have confirmed that most of the flexion–extension occurs at the poll and the base of the neck when the horse voluntarily performs dynamic mobilization exercises to end range of motion in cervical flexion (Clayton et al., 2010a) or in cervical lateral bending (Clayton, et al., 2012). There have been few published anatomical descriptions of the facet joints in the equine cervical spine, particularly with reference to the prevalence, clinical signs and pathological features of degenerative changes. Ultrasonographic imaging of the equine cervical region provided a reference for normal appearance of cervical vertebrae, facet joints, and paravertebral structures in eight horses of unspecified breed, between the ages of 2 and 14 years (Berg et al., 2003). A retrospective study of radiographic images of 122 horses concluded that the size of the caudal cervical facet joint at the level of C5–C6 increases with age but it is not known whether there is an association between such changes and clinical symptoms or performance (Down & Henson, 2009). Claridge et al. (2010) described normal anatomical shape, spatial orientation and joint volume for the cervical facet joints in six subjects. Little is known about cervical intervertebral disc disease in horses. The only study of the gross anatomy of the discs (Bullwein & Hannichen, 1989) involved evaluations of midline sections the cervical vertebrae without preparing histological sections. The authors saw no evidence of an annulus fibrosus or a nucleus pulposus and some discs showed disintegration of the tissue fibers. Cervical disc disease has been reported in horses, but is likely under-reported since it poses a diagnostic challenge that may be solved by modern imaging equipment. Information on disc structure and common pathological changes will facilitate the interpretation of images of the discs. The joints in the mid neck (C3–C4, C4–C5 and C5–C6) undergo considerably less motion in vivo even when the neck is fully flexed or extended (Clayton et al., 2010a). It seems likely, therefore, that the equine cervical intervertebral discs will vary in thickness. Future CT imaging studies may provide information about the size and structure of the discs that cannot be obtained from standard radiographic or ultrasonographic imaging. As discussed in Chapter 13, disorders and lesions affecting skeletal muscle and peripheral nerves are common in horses and adversely affect athletic ability (Cardinet & Holliday, 1979; Freestone & Carlson, 1991; Martin et al., 2000; Gregory, 2004). Frequently, muscular dysfunctions are secondary to underlying bone pathology in horses with back pain but may also be due to pathology of the muscles themselves or to a generalized muscular disorder (Valberg, 1999; Quiroz-Rothe et al., 2002). It is suggested that this is due to altered motor control as a result of the underlying lesion in the spine and/or due to peripheral joint disease with pain and inflammation which causes reflex inhibition of motor neurons resulting in weakness and atrophy of associated muscles (Young, 1993). Local muscle damage attributed to a poorly fitting saddle, for example, can also cause atrophy of the epaxial muscles (Gellman, 1998; Harman, 1999). Figure 17.2 visually shows three examples of asymmetrical hindquarter muscle development, or relative. The authors suggest objectively measuring muscle size using ultrasonography, which may be a reliable tool for comparative measures across time and during the rehabilitation process. Research is currently underway using this modality in the horse. Jeffcott (1980) surveyed 443 cases referred with TL disorders finding that 23.37% had evidence of epaxial muscle pain. Stubbs et al. (2010) reported that there was a relationship between muscle function and pathology via ultrasonographic and necropsy analysis where significant atrophy of the multifidus muscles was evident at the level and site/side of TL lesions in 22 racehorses. Valentine (2008) investigated the pathological findings in equine muscle (excluding PSSM) in 229 equids (217 horses and eight ponies of multiple breeds, three donkeys and one mule) over a 2.5-year period through necropsy and histopathology. Muscle lesions were present in 65% with the most common findings being chronic myopathic changes (15%), generalized muscle atrophy (13%), denervation atrophy (6%), and other lesions and pathologies that occurred less frequently (<5%) including myonecrosis, bone fractures, bacterial infections, muscle rupture, selenium deficiency, exertional rhabdomyolysis, intramuscular protozoa, neoplasia, injection site reactions, lymphocytic infiltrates, ring fibers, fiber splitting, and fat infiltrations. The aetiology was undetermined in 4% of cases. Hunt et al. (2008) performed an epidemiological study of myopathies in Warmblood horses in which the most common was PSSM (72/132 horses), followed by RER (7/132), neurogenic or myogenic atrophy (7/132) and non-specific myopathic changes (14/132). Thirty-two biopsies were normal. Recently there have been significant advances in understanding the etiopathogenesis underlying these disorders including recognizing the similarities between human and equine muscle diseases (Piercy & Rivero, 2004). Myopathies often present as gait abnormalities or overt RER, with slow improvements in clinical signs through dietary management and a regulated exercise routine as part of their rehabilitation (Hunt et al., 2008). Accurate patho-anatomical diagnosis of back pain is clinically challenging. It requires a lengthy clinical examination and multiple diagnostic procedures including radiography, ultrasonography, scintigraphy, local analgesia, kinematics and EMG imaging in the attempt to reach a diagnosis and increase the knowledge about the possible causes (Jeffcott, 1980; Steckel, 1992; Weaver et al., 1999; Denoix & Dyson, 2003; Peham et al., 2001; Peham & Schobesberger, 2006; Roethlisberger Holm et al., 2006; Gillen et al., 2009; Girodroux et al., 2009; Meehan et al., 2009; Fuglbjerg et al., 2010). Further, as previously stated, many horses have multiple osseous lesions that can be detected along the length of the vertebral column (Meehan et al., 2009; Girodroux et al., 2009; Gillen et al., 2009; Cousty et al., 2010; Stubbs et al., 2010). Post mortem studies have confirmed the presence of multiple types of osseous lesions at multiple sites; unfortunately these horses were unable to be examined ante-mortem to determine the relationship between the grades of lesions and pain and function (Townsend et al., 1986; Haussler & Stover, 1998; Haussler et al., 1999b). Thus it is still difficult to determine the relationship between clinical signs, diagnostic findings (the grade/severity of the lesions) and level of pain, as this is very variable between individual performances and horses (Haussler et al., 1999b; Meehan et al., 2009). The clinical signs associated with a variety of osseous lesions of the vertebral column and pelvis are poorly described, often non-specific and difficult to validate objectively, with a decrease in performance being the main complaint of the rider/owner. Clinical diagnosis is further complicated by the fact that horses vary in their response to pain and it is believed that temperament also plays a role (Jeffcott, 1999). Clinical signs of back pain in horses include both behavioral and physical signs including altered sensitivity to palpation, which may be described as decreased mechanical nociceptive threshold (MNT); sinking when placing the saddle on the horse, when securing the girth (‘girthiness’) or when the rider mounts (‘cold back’); resistance in work, for example, not wanting to trot, canter or rein back, refusing to jump or tail swishing; and/or lameness of one or more limbs without a cause or possible diagnosis (Martin & Klide, 1997; Haussler et al., 2006; Mills et al., 2007). Horses may express pain by fleeing or evasion; adopting an abnormal stance, gait or speed; vocalizing or showing signs of aggression during movement or manipulation; restlessness; swishing the tail; and muscle tension and tremors (Gregory, 2004; Mills et al., 2007). When back pain becomes chronic, muscular atrophy of the back can become visible (Fig. 17.2). Chronic pain, unlike acute pain, can be present without inflammation or noticeable tissue damage. Horses with chronic pain can be aggressive or evasive when they cannot react in their natural way by fleeing. This is why owners and/or riders sometimes believe their horse has a behavioral problem, as they are not able to identify the underlying painful process (Ridgway et al., 2005). In summary, the clinical signs often are unclear, so it is important to exclude other problems in order to arrive at a diagnosis of back pain. Microtrauma from chronic overuse because of poor saddle and tack fit (Harman, 1999), sub-optimal riding technique or an inappropriate training schedule can all predispose horses to back pain (Haussler, 2000). Objective assessment of musculoskeletal pain in horses is challenging (Casey, 2002). Local tenderness is the major manifestation of most, if not all, musculoskeletal pain (Vanderweeën et al., 1996). Tenderness in the axial skeleton has traditionally been assessed by manual palpation, although the interpretation of its outcome is highly subjective (Haussler et al., 2007). Nonetheless, palpation plays an important role in the clinical examination of cases with suspected neck and back muscle sensitivity. Therefore, both equine veterinarians and physiotherapists have included palpation in their physical examination protocol (Ranner et al., 2002; Denoix & Pailloux, 2005; Cauvin, 1997). This is obviously particularly important for clinical decision-making and evaluation of therapeutic intervention (Ashley et al., 2005). Pathological findings of the vertebral column identified on radiographs often do not correlate with the clinical findings (Gundel et al., 1997; Ranner et al., 2002). Thus, Ranner et al. (2002) concluded that palpation remains one of the most important clinical examination methods to determine whether or not a horse is suffering from neck and back muscle sensitivity or pain. Pain in the TL region of a horse is very complex as it can originate from various pain receptor structures. Afferent nerve endings are found in various types of connective tissue including fascia around muscle bundles, muscle spindles, joint capsules, tendons and ligaments, blood vessel walls and bone. Some of these receptors are nociceptors (pain receptors) and respond to noxious stimuli, including mechanical, thermal or chemical stimulation. Muscle pain may range from very intense sharp localized pain in the acute setting (for example, a muscle tear), to chronic muscle pain, which may be dull and diffuse. Referred pain from abdominal or thoracic organs can project onto different parts of the body including the back (Gregory, 2004). Although very difficult to quantify in the horse neurogenic and or referred pain may also be present which is well documented in the human literature. Macgregor and von Schweinitz (2006) identified equine myofascial trigger points with similar objective signs and electrophysiological properties to those documented in human and rabbit skeletal muscle tissue. Unfortunately, referred pain patterns and reproduction of the pain profile cannot be determined in animals. Comparative pain characteristics between muscular and ligamentous saline injections have been investigated, as these methods are commonly used to induce human and animal back pain (Tsao et al., 2010). Interspinous ligament injections produced pain of greater intensity and duration compared to injecting the paraspinal muscles in the normal human lumbar spine. Interestingly muscle pain was reduced with contracting and stretching the injected muscle, but this did not affect the pain produced from the ligament injection. The authors were also surprised that many of the subjects (n = 10) pointed to a region of pain 1–2 segments away from the injection site (Tsao et al., 2010). Pain works as a protective mechanism to prevent further tissue damage and to allow healing of wounds and damaged tissue. Pain cannot be objectively measured, because it has no units. Changes in heart rate, blood pressure, plasma cortisol and behavior can be helpful to identify and study pain (Ashley et al., 2005; Robertson, 2006). Examples of ways to subdivide pain are acute versus chronic, somatic versus visceral and physiological versus pathological, which occurs when the tissue is damaged and responds with inflammation (Robertson, 2002). In mammals, inflammation is the greatest source of pain (Gregory, 2004). During inflammation, the nerve endings that respond to noxious stimuli such as heat, pressure and chemical stimuli become sensitized. When normal, non-painful stimuli are applied to sensitized tissue, the patient experiences a painful sensation. In the human this is commonly referred to as ‘allodynia’ (Woolf & Mannion, 1999). Bussières et al. (2008) established the value of behavioral and physiological criteria by developing and validating a composite multifactorial pain scale (CPS) in an experimental equine model of acute orthopedic pain. Eighteen horses were allocated to control and experimental groups that received Amphotericin-B injection to induce pain in the tarsocrural joint and various forms of analgesia. Inter- and intra-observer reproducibility was good (0.8 < K < 1), with the key specific and sensitive behavioral indices being palpation of the painful area and the horse’s posture. Other less valuable signs were pawing the floor, kicking the abdomen and head movement. There was a statistical correlation between the CPS and both non-invasive blood pressure (p < 0.0001) and blood cortisol (p < 0.002). Van Loon et al. (2010) recently investigated the reliability and clinical applicability of applying a CPS to objectively monitor somatic and visceral pain under hospital conditions in 94 horses (control, acute and chronic surgical and non-surgical groups). CPS showed low baseline values in healthy animals with non-painful conditions and these were not affected when general anesthesia was the only intervention. Inter-observer reliability was very high (n = 23 horses; weighted kappa correlation coefficient, κ = 0.81). Horses with painful conditions responding well to analgesic treatment could be discriminated from horses that had to be euthanized on humane grounds because of painful non-responsive conditions. It was concluded that the CPS is a promising tool to potentially provide a basis for direct day-to-day assessment of pain status in equine patients with various painful conditions in the future. The CPS may be a useful subjective tool as an adjunct to other subjective/objective measurements during the rehabilitation process. Kinematic measurements describe stride and step timing, stride and step lengths, and intervertebral joint motion and the symmetry in different gaits. It is a useful tool in evaluating the effectiveness of therapeutic interventions like chiropractic manipulation (Faber et al., 2003, Gomez Alvarez et al., 2008; Haussler et al., 2010). Johnston et al. (2004) performed a kinematic evaluation of the back in horses without a history of back problems in order to develop a database on kinematics. Older horses were shown to have decreased flexion and extension of the TL junction in trot. Kinematic analysis can be a useful tool to identify horses with back pain because the movement pattern of the back changes so that dorsoventral flexion and extension at T13 and T17 at walk and at T17 and L1 at trot are significantly reduced compared to horses without back pain but there is no difference in lateral bending between horses with and without back pain. During walk, horses with back pain have a decreased axial rotation of the pelvis. Decreased back motion can also be present due to pathological conditions (Wennerstrand et al., 2004; Gomez Alvarez et al., 2007a, b), with the most significant differences being visible in walk rather than trot. This is likely due to the fact that the trot inherently has less intervertebral motion than the walk. However, these studies were performed only on a treadmill and further investigation is warranted on a circle, on varying gradients and under saddle in a larger number of subjects with different types of back pain/pathology. The ‘normal’ movement of the horse’s back has been investigated via EMG in conjunction with comparisons of locomotor kinematics and kinetics. Many factors contribute to locomotion including the muscular system (longissimus dorsi, iliocostalis, semitendinosus, biceps femoris, and gluteus medius), acceleration from the hind legs, gravity and the influence of the rider (Licka et al., 2004, 2009; Peham & Schobesberger 2006; Wakeling et al., 2007; Zaneb et al., 2009). Gluteus medius is considered an epaxial muscle as it covers the majority of the dorsal lumbar spine, with attachments onto the thoracolumbar fascia and iliocostalis ventrally. The anatomical epaxial musculature of the lumbar spine is displayed in Figure 17.3.
Rehabilitation of the locomotor apparatus
General introduction
Historical background: rehabilitation defined
Longevity and musculoskeletal disorders
Prevalence of neck and back pain
Patho-anatomical diagnosis
Functional assessment
Kinematics
EMG
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rehabilitation of the locomotor apparatus