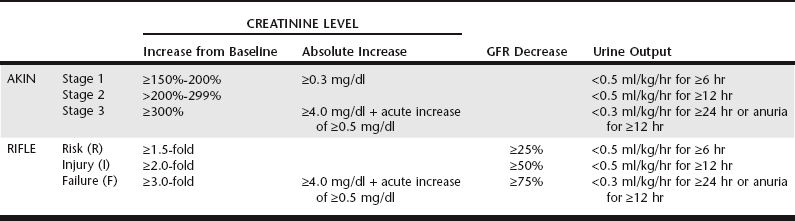
Chapter 186 There has been a shift in terminology in the human medical field within the last 10 years to establish a more concise and clinically relevant definition of acute renal failure (ARF). By the traditional definition, ARF is a rapid fall in glomerular filtration, which manifests clinically as an abrupt and sustained increase in the serum levels of urea and creatinine with an associated disruption of salt and water homeostasis. Since this definition cannot be quantified for clinical use, comparing incidence and outcome across clinical studies is challenging. To formulate guidelines to provide a more concise definition for ARF, physicians have proposed a definition of acute kidney injury (AKI) that includes reduction in kidney function within 48 hours defined by the occurrence of at least one of the following: (1) an absolute increase in serum creatinine concentration of at least 0.3 mg/dl (26.4 mmol/L), (2) an increase in serum creatinine concentration of more than 50% of baseline, or (3) oliguria of less than 0.5 ml/kg/hr for more than 6 hours. Through the use of two similar classification schemes to stratify human patients with regard to severity of AKI—the Risk-Injury-Failure-Loss-End-stage disease (RIFLE) criteria and the Acute Kidney Injury Network (AKIN) criteria (Table 186-1)—multiple retrospective and prospective studies have proven a significant association between increasing severity of AKI and mortality, even with minimal increases in creatinine concentration that previously were not considered important. TABLE 186-1 Comparison of Acute Kidney Injury Network (AKIN) and Risk-Injury-Failure-Loss-End stage disease (RIFLE) Criteria for Evaluating Hospital-Acquired Acute Kidney Injury Both AKI and HA-AKI have been investigated in the veterinary literature. In a retrospective review by Thoen and Kerl (2011) of data for 164 dogs admitted to the intensive care unit (ICU), 14.6% were noted to have developed AKI, as defined by an increase in creatinine concentration of at least 150% of baseline or an absolute increase of 0.3 mg/dl or more during the hospital stay. In another retrospective review (Harison et al, 2012), 15.2% to 19.3% of dogs for which at least two creatinine measurements were taken within a 2-, 3-, or 7-day period (n = 401, 477, and 646, respectively) developed AKI, as defined by an increase in serum creatinine concentration of 0.3 mg/dl or more. In both studies, only cases with serum creatinine levels of less than 1.6 mg/dl on the initial sample were included. There are no published prospective studies of the incidence of HA-AKI in dogs admitted to the ICU, but preliminary data suggest an incidence of around 10% to 17%. In cats, 20% to 21.3% of the at-risk population for which at least two creatinine measurements were taken within 2, 3, or 7 days (n = 128, 165, and 221, respectively) developed AKI, as defined by an increase in serum creatinine concentration of 0.3 mg/dl or more (Harison et al, 2012). Commonly recognized risk factors for HA-AKI include preexisting renal disease, dehydration and volume depletion, decreased cardiac output, advanced age, fever, hypotension, hypertension, hypoalbuminemia, sepsis, electrolyte imbalances, acidosis, hyperviscosity syndromes, liver disease, administration of nephrotoxic drugs, administration of radiocontrast media, pancreatitis, diabetes mellitus, anesthesia, and surgery. Of 29 dogs that developed HA-AKI, most had been exposed to a nephrotoxicant (72%); were older than 7 years of age (69%); had chronic heart disease (41%), preexisting renal disease (35%), a neoplastic condition (31%), or fever (28%); or had undergone anesthesia (14%) (Behrend et al, 1996). The nephrotoxicant drugs included aminoglycosides alone or in combination, cardiac drug combinations, cisplatin, and nonsteroidal antiinflammatory drugs. In contrast, almost 40% of 332 people who developed HA-AKI had experienced decreased renal perfusion (Nash et al, 2002). The causes of decreased renal perfusion included volume contraction, congestive heart failure, hypotension, cardiac arrest, inadequate blood pressure control, third-space losses, and arrhythmia. Other causes of HA-AKI were nephrotoxic medications, radiographic contrast media, postoperative status, and sepsis. Early recognition of HA-AKI is clinically useful for case management decisions as well as for prognosis. Unfortunately, renal injury can occur before the development of azotemia and uremia. Current and future studies of serum and urine biomarkers may define the clinical utility of measuring substances that indicate early or ongoing damage to nephrons. Urine biomarkers are biochemical substances in the urine that provide an indication of glomerular or tubular cell dysfunction and death. For example, veterinarians can measure the level of albumin in the urine, and in the absence of inflammation elevations in albumin level compared with urine creatinine level indicate glomerular injury (see Chapter 188). Low-molecular-weight proteins (LMWPs) are filtered freely by the glomerulus and under normal physiologic conditions should be reabsorbed completely by the proximal tubule; identification of LMWPs in the urine implies damage to the proximal tubular segments. One LMWP under investigation is urinary retinol-binding protein (URBP). In a recent study (Schaefer et al, 2011) dogs with naturally occurring systemic inflammatory response syndrome were found to have significantly increased urine protein : urine creatinine (UC) ratios, increased urine albumin to UC, and increased URBP to UC compared with healthy controls. The authors suggested that the presence of increased quantities of URBP could occur from direct tubular damage as an indication of AKI or from competition with other HMWPs for tubular binding sites. Further study is needed to determine the clinical significance of this urine biomarker. In addition to measurement of proteins, renal injury can be detected by measurement of urine enzymes. The enzymes used commonly for this purpose are large (>80 kD) and therefore are expressed only in the urine from leakage of damaged tubular cells. Enzyme measurements often are more sensitive than protein measurements because (1) enzyme levels will become elevated in urine before the onset of overt dysfunction, (2) analysis of urine enzymes often is easier than analysis of proteins, and (3) the amount of enzyme leaked may be predictive of the degree and severity of ongoing injury. The most common enzymes studied in human patients are glutathione S-transferases and enzymes located within the proximal tubule and brush border. N-acetyl-β-d-glucosaminidase (NAG) and γ-glutamyl transpeptidase are renal proximal tubular cellular enzymes. These enzymes have been measured in urine of healthy dogs to determine reference ranges. Maddens and colleagues (2010) evaluated NAG as well as urine protein biomarkers in bitches with naturally occurring pyometra and found increases in the ratios of all urine biomarkers compared with UC at the time of surgical management for pyometra. At 6-month postsurgical follow-up, urine biomarker levels had decreased and were similar to urine biomarker quantities in age-matched control dogs. Evaluation of a panel of multiple proteins and enzymes most likely will provide greater diagnostic accuracy in identifying and localizing renal injury.
Recognition and Prevention of Hospital-Acquired Acute Kidney Injury
Epidemiology and Etiology of Hospital-Acquired Acute Kidney Injury
Biomarkers of Acute Kidney Injury
Recognition and Prevention of Hospital-Acquired Acute Kidney Injury
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


