Alexis Lecu, Ray L. Ball
Recent Updates for Antemortem Tuberculosis Diagnostics in Zoo Animals
Tuberculosis (TB) and other mycobacterial diseases remain a difficult issue for veterinarian and zoo stakeholders to deal with. Regulatory requirements may give very broad obligations for prophylactic screening but are rarely precise enough to allow adequate follow-up with every zoo animal. Meanwhile, the risk of silent transmission between zoo animals and humans remains a real concern, as several cases of transmission are still reported every year from zoos and other institutions keeping captive wild animals all over the world.11,27,42,49,50 Within the last 5 years, more than 19 peer-reviewed publications dealt with single or grouped TB outbreaks in zoo settings, and more than 50 peer-reviewed publications were about the application of diagnostics methods in various wild animal species;11 this confirms that the research and tools currently available are improving greatly. It is imperative that zoo and wildlife clinicians have a basic understanding and access to these reference materials to choose between all recent methods and apply and interpret them properly. Although mycobacterial diseases will likely remain a challenge because of their complex pathophysiology, the zoo and wildlife practitioner has a larger, developing array of diagnostic options for both TB and non-TB mycobacterial screening. Most of the time, an indirect test alone is meaningless if it is not combined with clinical examination, direct screening, and with another indirect assay. Combinations of tests always improve the accuracy of TB diagnostics, as long as parallel tests that are independent of each other are chosen.
Direct Test
Although culture remains the gold standard to confirm the presence of mycobacteria, several tools are now available to get information indicating the presence of mycobacteria more quickly. Culture may be accelerated with the use of custom broth media associated with automated microbacteriologic detection systems such as the BACTEC Microbacteria Growth Indicator Tube (MGIT), which can produce results in 6 weeks with Mycobacterium tuberculosis or M.bovis.19 Mycobacterium DNA (deoxyribonucleic acid) sequences may also be revealed by molecular amplification techniques, which are now broadly available and have improved sensitivity values compared with those existing 10 years ago.24 Genotyping techniques are useful in understanding the epidemiologic origin and transmission of mycobacterial infections,47,48 and those techniques may sometimes be applied on raw samples (biopsies, fluids), prior to culture results, as long as sufficient mycobacterial DNA quantity is present. Intermittent shedding38,63 and low amounts of mycobacteria in internal fluids are likely to lower the sensitivity of the test, even if the molecular technique has excellent intrinsic sensitivity. Frequent collection such as repeated trunk washes in elephants38 is a way to overcome this limitation. The selection of an ideal sample should be based on clinical examination.34 Direct detection of mycobacteria in peripheral blood is a technique that is used especially for the diagnostic of extrapulmonary disseminated forms of TB.26 This technique demonstrates a sensitivity of 50% to 80%7,26 and has been recently applied in deer to investigate M. bovis or M. tuberculosis infection4 by using duplex and multiplex polymerase chain reaction (PCR) methods applied on EDTA (ethylenediaminetetraacetic acid) blood samples.
Indirect Test
Cell-Mediated Immunity
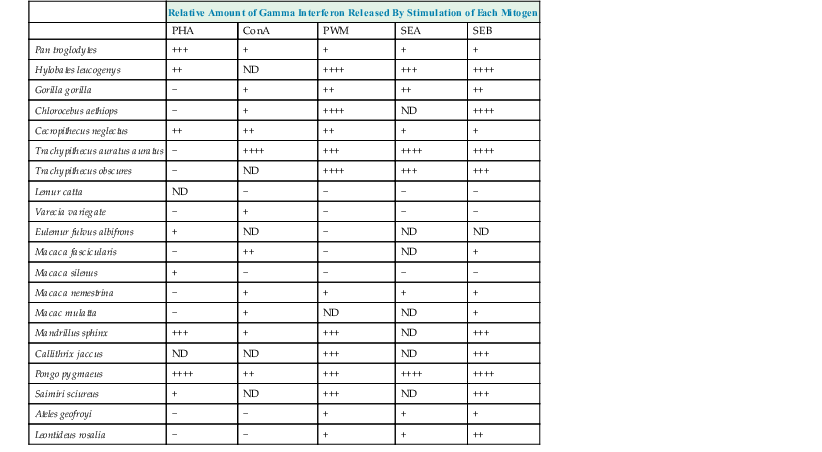
One of the most common antemortem indirect tests used over the years is the intradermal tuberculin skin test (TST), but its limitations have been already described in many wildlife species.9,45 Interferon gamma release assays (IGRAs) are now being used in veterinary medicine9,34 as an alternative to TST (Table 74-1). These tests measure in vitro cytokine (interferon-gamma [IFN-γ]) production by live lymphocytes contained in a fresh blood sample while exposed to selected mycobacterial antigens. The IGRAs measure the presence of an adaptive immune response to M. tuberculosis antigens and are thus only an indirect measure of exposure.31 The first advantage is that they are in vitro tests, which removes some of the variability of in vivo tests. Secondly, these tests could be further modified to bring more specific information by exposing blood cell samples to selected specific antigens from any Mycobacterium species. However, IGRAs have their own limitations that must be kept in mind when ordering them or interpreting their results.21 The animal IGRAs available commercially (e.g., Bovigam or Primagam) do not have positive controls, so they must then be selected and added into the tests by the operator.15 The choice of this mitogen will influence the result, as all interpretations of flowcharts are based on comparisons between the amounts of IFN produced by negative controls, antigens, and positive controls. A great discrepancy exists between mitogen stimulation abilities, according to the animal species,10,64 so the clinician should ask the reference laboratory about the molecule used as positive control or even suggest it according to the literature.
In commercial kits, enzyme-linked immunosorbent assay (ELISA) is used to detect the IFN-γ of cattle (Bovigam) or certain primate species (Primagam). As indicated in Tables 74-2 and 74-3, ELISA may be able to detect IFN-γ from a broader range of species.33 Moreover, use of a monoclonal IFN antibody designed for the targeted animal species may be used29,46 within these commercial IGRAs to improve the test quality (Table 74-4). RNA (ribonucleic acid) sequences coding for IFN-γ primers are now available for a wide range of animal species,6,61 with gene expression in peripheral blood mononuclear cells (PBMCs) of cattle5 or elephants58 used to distinguish infected animals from noninfected animals.
TABLE 74-2
Summary of Applicable Tests on Zoo and Wildlife Species
| Method of Detection | Test | Sample | Species | Pro | Con |
| Direct cellular immunity | PCR | Fluids (urine, BAL, discharges) Biopsies Histology slides | All | Molecular techniques | Contamination with other environmental mycobacteria |
| PCR | Blood | Screens mycobacterial antigens of highly disseminative forms | Mycobacteria itself rarely present within circulation | ||
| Culture | Biopsies, frozen tissues, fluids | Gold standard method Delay could be shortened by use of enhanced methods (see text) | Test turnaround is lengthy | ||
| Stain | Biopsies, fluids | Inexpensive | Limited by intermittent shedding | ||
| Indirect cellular immunity | Single tuberculin skin test | Soft, naked or shaved visible skin area. Should not be exposed to high temperature variation or external inflammation causes | NHP, Bo | Reduced cost | Poorer sensitivity and specificity Discrepancy among tuberculin Dosage is often empiric Reading test may require immobilization at 48–72 hours |
| Comparative tuberculin skin test | NHP, Bo, Cv | Enhanced specificity: could differentiate Mycobacterium avium or non-tuberculosis mycobacterial infection | Reading test may require immobilization at 48–72 hours | ||
| IGRA | Whole fresh (<8 h) blood on heparin | Bo, some OWM, Rh | Marketed for primates and cattle. Cattle test could be used with success in a lot of Bovidae and Giraffidae | Experimental rhinos and felids Not applicable to all primates Short viability of sample | |
| Indirect humoral immunity | ELISA | Serum | Ta, El, Cv, Rh, Bo | Test antibody to broad antigens (filtrate) or very specific ones (ESAT6, CFP10,…) Retrospective analysis | Cross reactivity to non-targeted species may confound results Host must mount humoral response |
| Rapid Lateral Flow Assays | Whole blood Serum (fresh or frozen | Bo, some OWM, El, Rh, Fe, Bo | Field use, results within 20 min Retrospective analysis | Host must mount humoral response |
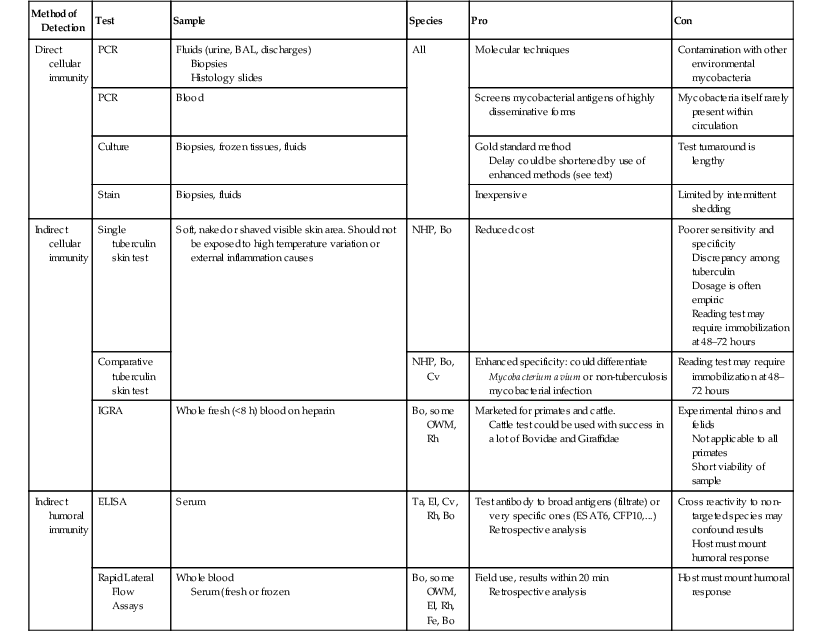
BAL, Bronchoalveolar lavage; ELISA, enzyme-linked immunosorbent assay; IGRA, interferon gamma release assay; NHP, nonhuman primate; OWM, Old World monkey; Bo, Bovidae; Cv, Cervidae; EL, elephant; Fe, felids; Pi, pinnipeds; PCR, polymerase chain reaction; Ta, tapir; Rh, rhinoceros.
From Schroeder B: Presented at Deutsches Primatenzentrum 01.12.2010 PrimagamThe Primate IFN-γ Test, Deutsches Primatenzentrum, Prionics AG; Wagistr 27A, 8952 Schlieren, Switzerland. Accessed December 23, 2013 http://www.dpz.eu/filadmin/content/Infektionspathologie/Bilder/Dokuments/SCHROEDER%20TB%20FORMAT%20Cop_Korr.pdf.
TABLE 74-3
Cross-Reactivity of Two Interferon-Gamma ELISAs
| Species | ELISA | |
| “Monkey” Interferon-Gamma | “Human” Interferon-Gamma | |
| Macaca rhesus | + | − |
| Macaca fascicularis | + | − |
| Macaca nemestrina | + | − |
| Papio sp. | + | + |
| Cercocebus athys | + | ND |
| Aotus sp. | + | + |
| Callithrix jacchus | − | + |
| Saimiri sciureus | ND | + |
| Pan troglodytes | ND | + |
ELISA, Enzyme-linked immunosorbent assay; ND, not done.
+ = detection/binding of species interferon.
− = detection failed.
From
TABLE 74-4
Interferon-Gamma Release Assays and Serologic Assays*
| Species | Commercial IGRA Brand Name Mitogen Used Stimulation Antigens (PPD) or (Re)combinant (ESAT6, CFP10) | Experimental IGRA Mitogen Used Stimulation Antigens (PPD) or (Re)combinant (ESAT6, CFP10) | Serologic Assay Comment† |
| Cattle: Domestic and wild (buffaloes, watusis) | Bovigam ConA Pokeweed PPD | ||
| Giraffes | Bovigam55 ConA Pokeweed PPD | ELEPHANT STAT PAK | |
| Bovinae, including Tragelpahinae | Bovigam55 ConA Pokeweed PPD | Elephant TB STAT PAK23 | |
| Reduncinae, including Kobus sp. | Bovigam55 ConA Pokeweed PPD | ||
| South American camelids | Based on pan-species IFN-γ ELISA Pokeweed30 PPD + REC | ENFERPLEX Vet TB STATPAK DPP38 | |
| Rhinoceroses | Under development(25) | Elephant TB STAT PAK23 MAPIA23 | |
| Elephants | Under development(53) | Elephant TB STAT PAK12 ELEPHANT DPP ® MAPIA12 | |
| Baboons | Primagam ConA55 PHA PPD | PRIMATB STAT PAK | |
| Macacas | Primagam ConA55 PHA, SEB PPD | PRIMATB STAT PAK | |
| Gorillas | Primagam Quantiferon Gold T SPOT TB58 PPD + REC | PRIMATB STAT PAK | |
| Orangutans | Primagam Quantiferon Gold T SPOT TB58 PPD + REC | PRIMATB STAT PAK | |
| Chimpanzees | Primagam Quantiferon Gold T SPOT TB58 PPD + REC | ||
| Pinnipeds | Elephant TB STAT PAK65 DPP MAPIA65 | ||
| Tapirs | Elephant TB STAT PAK65 DPP MAPIA65 | ||
| Deers | Based on pan-species IFN-γ ELISA or on IFN mRNA sequences detection by PCR Pokeweed PPD + REC | ||
| Meerkats | Elephant TB STAT PAK62 DPP MAPIA62 | ||
| Felidae | Based on monoclonal lion IFN-γ antibody PMA48 PPD + REC Not yet to confirm culture positive animals | Vet TB STATPAK48 Elephant TB STAT PAK73 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree