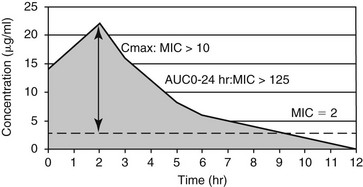
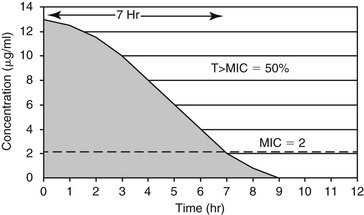
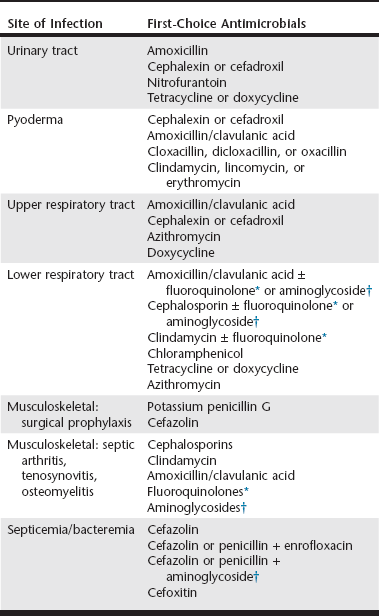
Chapter 260 Concerns regarding bacterial resistance to antimicrobials are increasing the awareness of their rational use in human and veterinary medicine. Empiric antimicrobial therapy requires matching the likely pathogens with the antimicrobial drugs that should be effective against them (Table 260-1). A successful empiric antimicrobial dosage regimen depends on both a measure of drug exposure (pharmacokinetics [PK]) and a measure of the potency of the drug against the infecting organism (pharmacodynamics [PD]). New information is emerging rapidly regarding the PK : PD relationships that determine antimicrobial efficacy in both human and veterinary patients (McKellar et al, 2004). The PK parameters used in drug dosage design are the area under the plasma concentration versus time curve (AUC) from 0 to 24 hours, the maximum plasma concentration (Cmax), and the time the antimicrobial concentration exceeds a defined PD threshold (T). The most commonly used PD parameter is the bacterial minimum inhibitory concentration (MIC). In relating the PK and PD parameters to clinical efficacy, antimicrobial drug action is classified as either concentration dependent or time dependent. TABLE 260-1 Suggested Empiric Antimicrobial Therapy by Site of Infection Based on the Likely Pathogens and Their Antimicrobial Susceptibility *Fluoroquinolones include enrofloxacin, orbifloxacin, marbofloxacin, and pradofloxacin, but only enrofloxacin is available in an injectable formulation. †Aminoglycosides include amikacin and gentamicin. In orthopedic infections, these drugs can be used in local therapy to avoid toxicity. For antimicrobials whose efficacy is concentration dependent, high plasma concentrations relative to the MIC of the pathogen (Cmax : MIC) and the area under the plasma concentration–time curve that is above the bacterial MIC during the dosing interval (area under the inhibitory curve, AUC0-24 hr : MIC) are the major determinants of clinical efficacy (Figure 260-1). These drugs also have prolonged postantibiotic effects, which allows once-daily dosing with maintenance of maximum clinical efficacy. For fluoroquinolones (enrofloxacin, orbifloxacin, pradofloxacin, marbofloxacin), clinical efficacy is associated with achieving either an AUC0-24 hr : MIC of more than 125 or a Cmax : MIC of more than 10. For aminoglycosides (gentamicin, amikacin), achieving a Cmax : MIC of more than 10 is considered optimal for efficacy. Other antimicrobials that appear to have concentration-dependent activity are metronidazole (Cmax : MIC > 10 to 25) and azithromycin (AUC0-24 hr : MIC > 25). These PK : PD targets are met when antimicrobials are administered at label dosages for the pathogens indicated on the label. For extralabel pathogens with high MIC values, such as Pseudomonas aeruginosa, achieving the optimum PK : PD ratios systemically may be impossible with label or even higher than label dosages. In such cases, underdosing is ineffective and merely contributes to antimicrobial resistance. For antimicrobials whose efficacy is time dependent, the time during which the antimicrobial concentration exceeds the MIC of the pathogen (T > MIC) determines clinical efficacy (Figure 260-2). How much above the MIC and for what percentage of the dosing interval concentrations should be above the MIC still is being debated and likely is specific for individual bacteria-drug combinations. Typically, exceeding the MIC by one to five multiples for between 40% and 100% of the dosing interval is appropriate for time-dependent antimicrobial agents. The time that the concentration exceeds MIC should be closer to 100% for bacteriostatic antimicrobials and for patients that are immunosuppressed. Therefore these drugs typically require frequent dosing or constant-rate infusions for appropriate therapy. An exception is cefovecin; this third-generation cephalosporin maintains concentrations above the target MIC for 7 days because of its high degree of protein binding. In sequestered infections, penetration of the antimicrobial to the site of infection may require high plasma concentrations to achieve a sufficient concentration gradient. In such cases, the AUC0-24 hr : MIC or Cmax : MIC, or both, also may be important in determining the efficacy of an otherwise time-dependent antimicrobial. The penicillins, cephalosporins, most macrolides and lincosamides, tetracyclines, chloramphenicol, and potentiated sulfonamides are considered time-dependent antimicrobials. Bacterial urinary tract infection (UTI) results when normal skin and gastrointestinal tract flora ascend the urinary tract and overcome the normal urinary tract defenses that prevent colonization. Large retrospective studies have documented the most common species of uropathogens in dogs and cats, with Escherichia coli being the single most common isolate in both acute and recurrent UTIs (Ball et al, 2008). Gram staining and determination of pH of a urine sample help direct empiric antimicrobial therapy. If the urine is persistently alkaline, a urease-producing pathogen such as Staphylococcus spp. should be suspected if Gram-positive cocci are seen, and Proteus if Gram-negative rods are seen. If the urine is acidic, the most likely pathogens are E. coli if Gram-negative rods are seen, and Enterococcus spp. or Streptococcus canis if Gram-positive cocci are seen. Initial treatment of uncomplicated UTIs is straightforward because most antimicrobials undergo renal elimination to a great extent, so urine concentrations may be up to 100 times peak plasma concentrations. All of the first-choice treatments are time-dependent antimicrobials, so frequent dosing is important for efficacy. Amoxicillin has excellent activity against staphylococci, streptococci, enterococci, and Proteus and can achieve high enough urinary concentrations to be effective against E. coli and Klebsiella if dosed frequently. Therefore amoxicillin every 8 hours is the most rational empiric therapy for bacterial UTI while culture and susceptibility results are pending. Amoxicillin/clavulanic acid has excellent bactericidal activity against β-lactamase–producing staphylococci, E. coli, and Klebsiella. However, clavulanic acid undergoes some hepatic metabolism and excretion, so the efficacy of amoxicillin/clavulanic acid may be due primarily to the high concentrations of amoxicillin achieved in urine; therefore it is not preferred over amoxicillin for first-line therapy.
Rational Empiric Antimicrobial Therapy
Concentration-Dependent Antimicrobials
Time-Dependent Antimicrobials
Urinary Tract Infections
Rational Empiric Antimicrobial Therapy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree