Barbara A. Byrne, Consulting Editor Antimicrobial stewardship is defined as “an activity that includes appropriate selection, dosing, route, and duration of antimicrobial therapy” in order that a positive clinical outcome can be achieved with a minimum of unintentional consequences.1,2 The dearth of new antimicrobial drug development with the advent of increasingly resistant bacteria necessitates that we use these drugs in a judicious manner that prolongs their effectiveness. Not only does inappropriate use of antimicrobial drugs result in the emergence of resistance to these drugs, they also can have such an impact on the normal microbiota that severe and sometimes fatal gastrointestinal disease can develop. Hence, veterinarians have an obligation to use these drugs in a responsible manner. Antimicrobial therapy is the cornerstone for treatment of infectious diseases. Therapy depends on appropriate identification of the infectious agent, determination of antimicrobial susceptibility of the causative organism, and application of pharmacokinetic/pharmacodynamic principles to achieve therapeutic levels of drug at the site of infection. Additional factors such as route of administration, toxicity, cost, compliance with administration, penetration of the drug of interest to the site of infection, host factors, and development of resistance must be considered when choosing an antimicrobial drug regimen (Box 45-1). Avoiding development of resistant pathogens or commensal flora is also desirable. This chapter focuses on use of antimicrobial drugs directed toward bacterial pathogens; however, similar principles apply when choosing antifungal, antiparasitic, or antiviral therapies. The ultimate goals of antimicrobial therapy are to provide effective treatment, with the fewest adverse reactions and at the lowest cost, and to prevent development of resistance in the target organism and host microbiota. Indiscriminant or inappropriate use of antimicrobial agents leads to development of resistance in the targeted pathogen as well as the host microbiota. The consequences of resistance are many-fold, including the inability to treat certain infections, superinfections, and transfer of resistant organisms to humans.3 As veterinarians, we have an ethical obligation to protect both animal and human health and well-being through judicious use of antimicrobial drugs. A consensus statement published by the American College of Veterinary Internal Medicine3 provides guidance for use of antimicrobial drugs and raises important considerations for the veterinarian regarding their use. One recommendation of this consensus statement is that the veterinarian develops standardized antimicrobial drug use guidelines for different types of cases (e.g., wounds, respiratory infections) that are appropriate for their hospital or clinical practice.3 These authors also indicate that antimicrobial therapeutics be categorized into primary-, secondary-, and tertiary-use drugs. Primary-use drugs are older antimicrobials that have a narrower spectrum of activity (e.g., penicillin, older tetracyclines, and sulfonamides) and are used for most infections. Secondary-use drugs generally have a broader spectrum than primary-use drugs and are reserved for more serious infections or when susceptibility data indicate resistance to primary drugs. Tertiary-use drugs are those with an even further extended spectrum of activity, are effective against resistant bacteria, and are also important drugs used for human patients. These tertiary drugs should be reserved for serious infections with confirmed resistance to all primary and secondary drugs. Thus, use of higher-level drugs should be directed by antimicrobial susceptibility testing. In addition to establishing tiers of drugs for antimicrobial use, it is important to follow a logical approach when deciding on the type of antimicrobial drug to use. This starts with establishing whether an infection is present, identification of the organism(s), determination of antimicrobial susceptibilities, and consideration of host factors. Once therapy has been initiated, assessment of the patient is essential to decisions regarding length of treatment and whether antimicrobial therapy should be altered. In addition, adjunctive therapies, such as surgical interventions should be considered. This is a frequently overlooked step for consideration when determining what type of antimicrobial therapy should be instituted. Too often, antibacterial drugs are used “just in case” there is an infection without proper examination for the signs of infection. A number of clinical signs and clinical laboratory findings can be evaluated that may point to the presence of an etiologic agent. The old saying that redness, swelling, and heat are cardinal signs of infection and inflammation still holds true. Certainly one frequently encountered finding is elevation of body temperature. However, inflammation for reasons other than the presence of an infectious agent may result in fever. Swelling and redness are indications of a localized infection. Development and appearance of purulent material is further evidence of infection. Clinicopathological findings are also important supportive evidence for an infectious disease. Elevated or depressed peripheral white blood cell counts frequently accompany significant infections. In large animal species, fibrinogen elevations are observed in bacterial infections. Serum biochemistry values may also inform the decision to use antimicrobial therapy. For example, elevations in globulin may indicate production of excess immunoglobulin as seen in subacute to chronic infectious, primarily bacterial, processes. Decreases in serum albumin may develop as a negative acute phase protein in response to inflammation accompanying an invasive pathogen.4 If possible, cytologic examination of material from the site of suspected infection should be performed. The presence of neutrophils, particularly with features of degeneration, is indicative of a septic process. Observation of infectious agents, ideally intracellularly, confirms the presence of an infection. Once the presence of an infection is determined, the next step is to decide what organism might be present. Ideally, samples should be collected prior to initiating therapy so that an etiologic diagnosis can be made and susceptibility testing performed. In the case of a serious or life-threatening infection, therapy must be initiated prior to receipt of culture and susceptibility results. Empirical therapy often relies on clinical judgment and knowledge regarding the likely organisms present depending on the body system affected and their likely susceptibility pattern. Additional findings such as results from cytologic examination help to inform the antimicrobial choice. Appropriate collection and transport of samples to the microbiological laboratory are essential to detection of the causative agent. The site of the infection should be sampled. This may seem obvious; however, a surprisingly large number of inappropriate samples are submitted to the microbiology laboratory. For example, sampling of nasal discharge is almost never the appropriate choice when faced with purulent nasal discharge. Identification of the large number of normal bacteria and interpretation of the findings are difficult when normally colonized sites are sampled. The actual source of the discharge, such as the sinus, guttural pouch, or lung should be determined and sampled directly. An exception to this rule is in the case of equine Streptococcus equi subsp. equi infections. In general, sterile sites are the best regions to sample. Lesions such as a draining tract are problematic. Frequently, they have superficial contamination of the opening of the tract that is inadvertently sampled. When possible, underlying soft tissue or bone should be collected rather than inserting a swab into the tract. If anaerobic culture is deemed necessary, it is important that adequate volume of tissue or fluid be collected to preserve the anaerobic environment. If possible, at least a milliliter of fluid or a square centimeter of tissue should be collected. Swabs are inappropriate for anaerobic culture because air can be trapped between the fibers, leading to death of non-aerotolerant obligate anaerobes. If the sample is smaller and not taken immediately to the laboratory, it should be placed in some sort of transport media that will maintain an anaerobic environment. These anaerobic systems will maintain the presence of aerobic and facultative anaerobes as well. Samples for microbiological culture should be transported to the laboratory as soon after collection as possible. The sample should be refrigerated if it cannot be delivered quickly, and it should be kept cool or cold but not frozen for transport to the laboratory. An exception to this rule is when anaerobic transport media (e.g., Anaerobe Systems, Morgan Hill, Calif.) is used. These vials should be maintained at room temperature. It is best if samples are collected prior to initiation of therapy. However, if antimicrobial treatment has already been instituted, the clinician should consider withdrawing therapy for a short period of time before sampling. Between 24 and 36 hours after the last administration of drugs is advisable, and 99% of the drug should be eliminated after 7 half-lives. In seriously ill animals such as the septic foal, delaying antibiotic therapy for any amount of time to collect samples may be detrimental. In such cases, samples should be collected just prior to the next administration of drug. Some blood culture systems contain resins that bind or inactivate antimicrobial drugs and have proven to increase the ability to detect bacteria within the sample.5 Initial examination of a sample provides important clues to the type of organism present. A Wright-Giemsa–type stain is frequently used to examine cytologic preparations made from samples. This staining technique can provide much information regarding the bacterial or fungal organism present. Identification of coccoid or rod-shaped bacteria along with the site of infection will often result in a differential diagnosis list of organisms to consider. An examiner with a practiced eye may be able to make an educated guess about the genera of the organism observed. A Gram stain provides additional information. Although many veterinarians do not use this stain in their clinical practice, this technique is rapid and, with a little practice, easy to perform. Identification of an organism as gram negative or positive further informs the practitioner of the likely organisms present and helps to guide empirical antimicrobial choice (Table 45-1). One of the first decisions to be made when considering antimicrobial therapy is the severity of the infection. The clinician needs to decide if therapy must be initiated immediately or whether the patient can wait for results of culture and susceptibility testing before initiating therapy. Obviously, life-threatening infections require immediate and aggressive antimicrobial therapy. Infections of sites such as an equine joint may necessitate empirical therapy while awaiting the results of microbiological testing. In this case, samples should be taken for identification and susceptibility testing before initiating therapy. On the other hand, an animal with a chronic, mild, or moderate infection may wait to receive antimicrobial drugs until testing has been completed. In a perfect world, the clinician should have laboratory-generated antimicrobial susceptibility results to guide therapy. However, frequently therapy has to be initiated prior to receipt of culture and susceptibility results. Empirical therapy should be based on knowledge of the inherent resistance of suspected agents and the antimicrobial susceptibility patterns observed in the practice region. Regular generation of susceptibility data should be compiled by the microbiology laboratory and used by the practitioner in the hospital setting. The microbiology laboratory should be proficient in antimicrobial susceptibility testing and follow guidelines established by the Clinical Laboratories Standards Institute (CLSI).6 Quality assessment testing should be performed regularly. A number of methodologies are used to examine susceptibility to different antimicrobials. Two frequently used methods for antimicrobial susceptibility testing are disk diffusion, also known as Kirby-Bauer, and broth dilution or broth microdilution, which uses a 96-well plate. Disk diffusion is a relatively straightforward testing methodology that can be performed with little specialized equipment. It involves creating a lawn of bacteria using a standardized amount, placing disks containing a set amount of antimicrobial drug on top of the bacterial lawn, incubating, and measuring the zone of inhibition around the disk. This zone diameter determines whether the bacterium should be considered susceptible, intermediately susceptible, or resistant to the particular drug. The advantages of this method are that it is rapid and easy to perform provided strict protocols are followed. The disadvantages are that it cannot be used for slow-growing bacteria and that it provides only an interpretation rather than information about the specific concentrations of drug that inhibits growth. Consequently, knowledge about the pharmacokinetics and pharmacodynamics of a particular drug cannot be fully used to develop a treatment regimen to deliver optimal dose of drug to the site of infection. The broth dilution or microdilution method involves inoculation of a standard amount of bacteria into tubes or wells containing an antimicrobial drug. Most clinical laboratories utilize twofold dilutions of drug centered on the amount of drug found systemically. The plate is subsequently incubated and then the wells are examined for visible growth. The lowest concentration of drug that inhibits visible growth is called the minimum inhibitory concentration (MIC). Sometimes the wells are subcultured to a nonselective agar and then incubated, and this agar is examined for growth. The lowest concentration of drug that results in no growth is the minimum bactericidal concentration (MBC). Because of cost reasons, most clinical laboratories do not determine the MBC for each organism. The MIC is unique for each bacterium : drug combination. The resulting MIC guides interpretation of the bacterium as susceptible, intermediately susceptible, or resistant to the particular drug. The concentration of drug at which the interpretation of testing changes from susceptible to intermediate or resistant is termed the breakpoint. For example, bacteria are considered susceptible to amikacin if the MIC is less than or equal to 16 µg/mL; they are deemed resistant if the MIC is greater than or equal to 64 µg/mL. If the MIC is 32 µg/mL, the isolate is considered intermediately susceptible.6 Frequently, the clinician only needs to use the interpretation when deciding which drug to choose for therapy. However, the MIC can be used to refine therapy, through increasing the dose of drug or frequency, to optimize treatment. Interpretation breakpoints for susceptibility testing of a bacterium as susceptible or resistant are set by the CLSI. This body bases the interpretation on three major factors: the epidemiologic breakpoint of a population of bacteria, pharmacokinetic data, and, when available, clinical outcome from treatment of animal or human patients.7 A graph showing MICs of a population of a single species of bacteria frequently has a bimodal distribution. The trough between the two peaks is the epidemiologic breakpoint; those bacteria with higher breakpoints have acquired some sort of resistance determinant. The pharmacokinetic information used to assist with determination of the breakpoint includes the peak concentration of drug, the area under the pharmacokinetic curve, and time the drug is above a particular MIC. A simplified way to think of how these data are used is to look at a bacterium with a high MIC. It would be considered resistant because the amount of drug administered in order to exceed the MIC would be considered toxic to the animal host. Generally, the highest concentration of drug tested is near or a bit above the highest concentration of drug that can be achieved with recommended dosages. Antimicrobial therapy should be directed to achieve levels that are above the MIC for the particular isolate. Ideally, the “best” antimicrobial drug to use depends on many factors other than the susceptibility result. Obviously, the organism should be susceptible to the drug chosen but other considerations should be included such as route of administration, penetration to the site of infection, toxicity, cost, and convenience. All of these factors being equal, the optimal choice of a drug is one in which the organism’s MIC is several dilutions below the breakpoint. However, further refinement is possible when one understands the pharmacodynamic properties of the drug and bacterium. The terms bactericidal and bacteriostatic refer to the in vitro observation that a drug kills or merely inhibits growth of a particular bacterium, respectively. If one examines the relationship between the MIC and MBC, these two values are close or nearly identical for bactericidal drugs. In contrast, the MBC is several twofold concentrations above the MIC for bacteriostatic drugs. To be clear, it takes a higher drug concentration to kill bacteria with a static drug. The in vivo consequence of this relationship means that more drug must be delivered to the site of infection in order to kill the bacteria with a bacteriostatic drug. Often this is not achievable given the toxicity and pharmacokinetics of the drug. In the case of bacteriostatic drugs, the clinician must rely on the host immune responses to clear the inhibited organisms. Consequently, bactericidal drugs should be used in an immunocompromised patient. Some drugs are concentrated in areas of infection. For example, drugs undergoing renal clearance are found in high concentrations in urine. Thus, drugs that show in vitro bacteriostatic activity may achieve concentrations high enough to kill bacteria in the bladder. The converse is also true; use of a bactericidal drug at inadequate dosages or that have poor penetration to the site of infection may not achieve high enough concentrations to kill the offending organism. Pharmacokinetic (PK) and pharmacodynamic principles can also be used to guide antimicrobial therapy. The relationship between the MIC and PK parameters such as the peak drug concentration may also determine the outcome of therapy. There are two categories of antimicrobial drugs: concentration-dependent and time-dependent drugs. This categorization is also primarily based on in vitro work but is supported by clinical data in animals and humans.8 Concentration-dependent drugs are those whose antimicrobial activity is best predicted by the relationship between peak drug concentration and the MIC of the organism. Drug classes that are considered concentration-dependent drugs include aminoglycosides and fluoroquinolones. The ratio between the peak drug concentration and the MIC should equal at least 8 to 10.9 Using the example of amikacin given earlier, if the bacterium had an MIC of 2 µg/mL, the drug should be dosed to reach a peak concentration of 20 µg/mL. The time that the drug concentration is above the MIC appears to be less important for concentration-dependent drugs. Accordingly, many concentration-dependent drugs can be administered infrequently (e.g., once daily). This dosing strategy can be advantageous with certain drugs with toxicity. For example, nephrotoxicity of the aminoglycosides is best determined by trough concentrations of drug. These antimicrobials may be administered at high dosages once daily to achieve the high peak concentration, well above the MIC, while having prolonged low trough levels. Many concentration-dependent drugs have prolonged post-antibiotic effect, meaning that the effect of the drug to inhibit growth of the organism is apparent for some time after the drug is removed. Fluoroquinolone efficacy is best predicted by the area under the concentration curve (AUC) in relationship to the bacterial MIC. The AUC : MIC ratio should be equal to or greater than 125 for gram-negative organisms.8–11 This ratio should be 60 or greater for gram-positive bacteria.8 Some studies indicate that ratios that approach 250 or more be used to avoid development of resistance.8 Time-dependent drugs are those whose antimicrobial activity is best predicted by time that the drug concentrations are above the MIC. Many drug classes are considered time dependent, including the beta-lactam drugs and tetracyclines. These drugs should remain above the MIC for at least 50% of the time. These antimicrobial drugs generally are dosed more frequently or via continuous rate infusion for those with short half-lives. Knowledge of the pharmacodynamic principles such as the peak drug concentration : MIC ratio or time above the MIC can guide the practitioner in appropriate dosages and frequencies for an individual drug. Monitoring of drug levels can further aid development of the therapeutic plan. Table 45-2 summarizes the pharmacodynamic parameters and activities of some example antimicrobial drugs.12 TABLE 45-2 Antimicrobial Drugs, Classifications, Activities, Post-antibiotic Effect, and Pharmacodynamic Parameter * Yes = prolonged effect longer than 6 hours; no = less than 1 hour. † T > MIC, time drug concentration is greater than the MIC; AUC24/MIC, area under the concentration curve over 24 hours/Minimum inhibitory concentration; Cmax/MIC, Maximum concentration/Minimum inhibitory concentration. Adapted from Martinez M, Toutain PL, Walker RD. 2006. The pharmacokinetic-pharmacodynamic [PK/PD] relationship of antimicrobial agents, pp 81-106. In Giguere S, Prescott JF, Baggot JD, et al (eds), Antimicrobial therapy in veterinary medicine, ed 4. Blackwell Publishing, Ames, IA. Too often the question of where the infection is located is not considered when choosing an antimicrobial drug. Penetration of the drug to the site of infection is essential for successful therapy. Remember that the MIC and interpretation of the MIC are focused on systemic (plasma) levels of drugs and rarely include the subtleties of drug penetration to various sites. Some anatomic locations are particularly difficult to enter, such as the central nervous system, ocular fluid, and bone. Abscesses are another structure that is difficult for antimicrobial drugs to penetrate; they are surrounded by a thick capsule with poor blood supply and lack of oxygen, which can limit penetration into the abscess and offer poor entry into the bacteria. The pH is generally low, which may inactivate some agents, and there is abundant protein present to bind antimicrobial drugs. A further complication is the presence of purulent debris, which can provide metabolic precursors for bacteria to bypass inhibitors of metabolism, such as trimethoprim-sulfa combination drugs. In general, lipophilic drugs will be better able to penetrate these difficult-to-reach anatomic locations. Highly polar or charged molecules have difficulty reaching these sites. If the causative organism is found intracellularly as seen with rhodococcal pneumonia or as with Corynebacterium pseudotuberculosis infections, the cell membrane is an additional impediment to drug penetration. Fortunately, some drugs penetrate cells well, such as macrolides, rifampin, chloramphenicol, fluoroquinolones, and trimethoprim-sulfa combinations. Empirical therapy is used for severe infections prior to obtaining culture and susceptibility results. The choice of antimicrobial drug depends on the clinician’s knowledge of the likely agents present at the site of infection and their predicted susceptibilities. Knowledge of the Gram-staining results can further guide therapy. Generally, empirical therapy is broad spectrum to cover multiple possible etiologies. Eventually, once the etiologic agent and its susceptibility have been identified, definitive therapy, using a drug(s) with a narrower spectrum, can be initiated. Definitive therapy should be directed to best result in a cure, using the narrowest spectrum of drug therapy, and have limited toxicity for the patient. The goal is to de-escalate the spectrum of activity of the drug as much as possible with definitive therapy in order to minimize the impact on commensal bacteria and development of resistance. Many infections can be effectively treated with a single antimicrobial drug; however, in some instances, combination therapy may be chosen. Some drug combinations have been demonstrated to be synergistic based on both in vivo and in vitro studies. Synergy is seen when the ability of the combination to kill bacterial growth is greater than the mere additive effect of each drug. Drug combinations can also be indifferent, where each drug’s effects are merely additive. Finally, a drug combination may be antagonistic where the drug combination effectiveness is less than the sum of the independent activities. Synergistic antimicrobial drug combinations are best used to treat resistant organisms. A common synergistic pair of antimicrobial drugs can be seen in the combination of trimethoprim with a sulfonamide. Either agent alone would be considered bacteriostatic, but since each of these drugs inhibits sequential steps in folic acid metabolism, they are considered bactericidal. A combination of antimicrobial drugs might be chosen for empirical therapy before the agents and their susceptibilities are known in order to provide broad-spectrum therapy. The combination of a β-lactam drug with an aminoglycoside is frequently chosen as empirical therapy in equine patients. This combination is synergistic as well as broad spectrum. It is important to remember that in these cases, therapy should be reduced to a single agent or a less broad spectrum of activity as soon as testing results become available. The presence of a polymicrobial infection is another reason why combination therapy may be used because a single agent will not be able to treat all bacteria present. For example, intraperitoneal infections are frequently caused by more than one agent. It is difficult to find a single antimicrobial drug to treat all the possible bacteria present such as enterics, gram-positives, and anaerobes. Individual drugs are available for use in humans that are sufficiently broad spectrum; however, many of these would be cost prohibitive in large animal patients. The clinician should be cautious, however, of isolation of multiple agents from a single site such as a wound. Superficial samples taken from these sites may have contaminating bacteria, often with highly resistant antimicrobial patterns, that are not truly part of the infectious process. Combined antimicrobial drug therapy can be used to prevent emergence of resistant bacteria. Certainly for serious or difficult-to-treat infections such as those caused by Pseudomonas spp., avoiding development of resistance is important not only to the patient receiving treatment, but also to others who come in contact with it. Interestingly, the ability of combination therapy to prevent resistance seems to be important only when it results from chromosomal mutations.13 The best illustration of this principle is in the treatment of human patients for Mycobacterium tuberculosis, in which combination therapy is always used to prevent development of resistance.13 There are some drawbacks to using combination therapy. One is the possibility of antagonism between antimicrobial drugs. Most instances of antagonism have been demonstrated in vitro, although in vivo reports have appeared.13,14 This phenomenon has been seen between gentamicin and chloramphenicol to treat Proteus mirabilis infections in immunocompromised mice.15 Use of antimicrobial drug combinations is also an increased cost to the client. Finally, there is an increased possibility of toxicity of and/or hypersensitivity reactions to the drugs when used together. Antimicrobial drug combinations that may be detrimental include penicillin with tetracyclines and chloramphenicol with erythromycin. Combination therapy should not be chosen merely to provide comfort to the clinician but rather clinicians should have specific reasons for their choice. Drug incompatibility can be the result of direct incompatibility, meaning that they should not be mixed together for administration or that they should not be used together therapeutically. Table 45-3 provides information about antimicrobial drug incompatibilities. TABLE 45-3 Specific Drugs and Additives That Are Incompatible When Comixed before Administration38,39 The age of the animal may influence many aspects of antimicrobial therapy. Bioavailability of certain drugs varies with age where some drugs can be used orally in neonatal or young animals but are poorly absorbed in mature animals.16 The drug distribution may also vary with age because young animals generally have a larger volume of distribution than adults because of their larger percentage of total body water.17 Drug biotransformation in neonates may vary from that in adults as well.17 Therefore, drug dosages and/or frequencies may have to be adjusted in young animals to provide adequate levels of drug at the site of infection and to minimize toxicity. Fluoroquinolone use in young and adult animals should be considered carefully. This class of antimicrobial drug has been documented to cause cartilage damage in several species of young animals, and ciprofloxacin has been associated with tendinopathies in humans.18–20 Of interest is the observation that fluoroquinolones are capable of having chondrotoxic effects on equine and canine chondrocytes in vitro,21 whereas a lamb model failed to demonstrate chondrotoxicity.22 Unfortunately, specific guidelines for safe use of fluoroquinolones in young animals are lacking. If fluoroquinolone use is necessary in young animals, it is important to limit the duration of therapy in order to minimize the chances of damage to developing cartilage. Along the same line, tetracyclines should also be avoided in young patients to avoid discoloration of enamel of developing permanent teeth. Antimicrobial drugs may have deleterious effects on reproduction and the developing fetus. Depending on the drug, the type of placentation, and stage of gestation, some drugs are able to reach the fetus. For example, use of potentiated sulfonamides in pregnant mares has been associated with anemia, fever, and abortion.23 Administration of pyrimethamine and trimethoprim-sulfamethoxazole to stallions has been associated with weakness and abnormal copulatory form and function.24 The lactating large animal must also be considered. Many antimicrobial drugs can be found in milk and may be transferred to the lactating animal or human who consumes the milk. Some drugs reach the milk in high concentrations whereas others are found in low amounts relative to plasma. Regardless, almost all antimicrobial drugs may be found in milk. Such adulteration may make the milk unsuitable for human consumption, and appropriate withdrawal times should be adhered to before milk enters the human food chain. Treatment of bacterial mastitis provides several challenges and is covered in more detail elsewhere in this book. Treatment should be initiated early and based on susceptibility of the particular causative agent. If individual milk culture and susceptibility testing is not feasible, then treatment should be based on historical information from the affected herd. Penetration into milk is also an important consideration when using systemic drugs to treat mastitis. Many drugs are found in much lower concentrations in milk, such as the β-lactams and sulfonamides. Others can be concentrated into milk, such as the macrolides. Because of the low concentrations found in milk for many of the drugs used in food production animals, intramammary administration is preferred, although systemic administration can also be used and is probably indicated in the systemically ill animal or when abscesses preclude delivery of the drug via the intramammary route. As for systemic administration, withdrawal times should be closely followed with intramammary infusions.
Rational Antimicrobial Therapy

Antimicrobial Stewardship
Is There an Infection?
What Organism Is Present?
Sample Selection and Transport to the Laboratory
Microscopic Examination of Sample
What Is the Severity of the Infection?
What Is the Likely or Confirmed Antimicrobial Susceptibility of the Organism?
Bactericidal and Bacteriostatic Antimicrobial Drugs
Concentration and Time-Dependent Antimicrobial Drugs
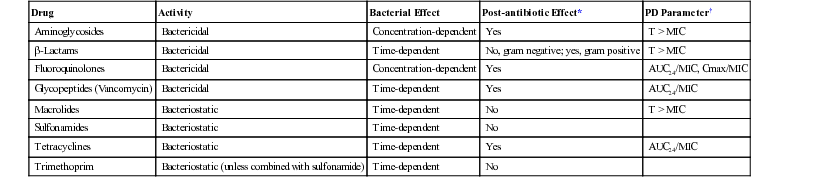
Drug
Activity
Bacterial Effect
Post-antibiotic Effect*
PD Parameter†
Aminoglycosides
Bactericidal
Concentration-dependent
Yes
T > MIC
β-Lactams
Bactericidal
Time-dependent
No, gram negative; yes, gram positive
T > MIC
Fluoroquinolones
Bactericidal
Concentration-dependent
Yes
AUC24/MIC, Cmax/MIC
Glycopeptides (Vancomycin)
Bactericidal
Time-dependent
Yes
AUC24/MIC
Macrolides
Bacteriostatic
Time-dependent
No
T > MIC
Sulfonamides
Bacteriostatic
Time-dependent
No
Tetracyclines
Bacteriostatic
Time-dependent
Yes
AUC24/MIC
Trimethoprim
Bacteriostatic (unless combined with sulfonamide)
Time-dependent
No
Where Is the Infection?
Empirical versus Definitive Therapy
Combination Therapy
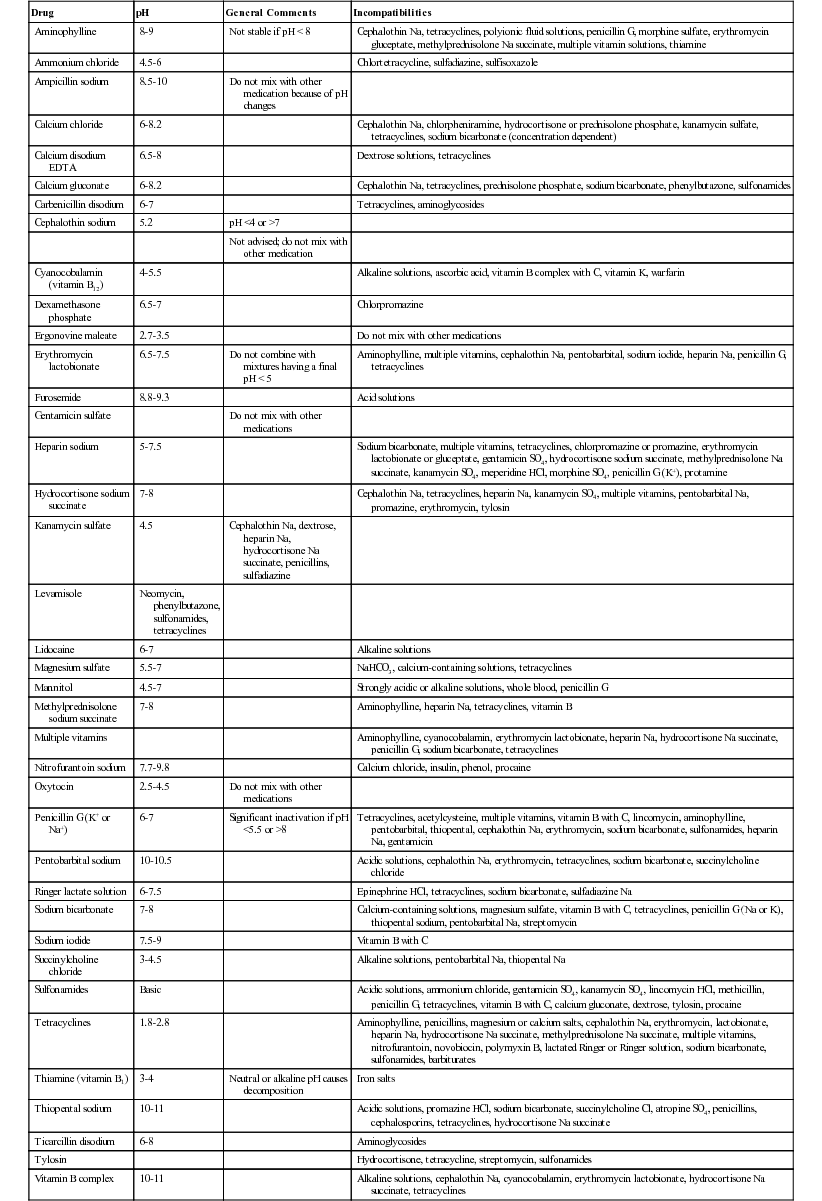
Drug Incompatibility
Drug
pH
General Comments
Incompatibilities
Aminophylline
8-9
Not stable if pH < 8
Cephalothin Na, tetracyclines, polyionic fluid solutions, penicillin G, morphine sulfate, erythromycin gluceptate, methylprednisolone Na succinate, multiple vitamin solutions, thiamine
Ammonium chloride
4.5-6
Chlortetracycline, sulfadiazine, sulfisoxazole
Ampicillin sodium
8.5-10
Do not mix with other medication because of pH changes
Calcium chloride
6-8.2
Cephalothin Na, chlorpheniramine, hydrocortisone or prednisolone phosphate, kanamycin sulfate, tetracyclines, sodium bicarbonate (concentration dependent)
Calcium disodium EDTA
6.5-8
Dextrose solutions, tetracyclines
Calcium gluconate
6-8.2
Cephalothin Na, tetracyclines, prednisolone phosphate, sodium bicarbonate, phenylbutazone, sulfonamides
Carbenicillin disodium
6-7
Tetracyclines, aminoglycosides
Cephalothin sodium
5.2
pH <4 or >7
Not advised; do not mix with other medication
Cyanocobalamin (vitamin B12)
4-5.5
Alkaline solutions, ascorbic acid, vitamin B complex with C, vitamin K, warfarin
Dexamethasone phosphate
6.5-7
Chlorpromazine
Ergonovine maleate
2.7-3.5
Do not mix with other medications
Erythromycin lactobionate
6.5-7.5
Do not combine with mixtures having a final pH < 5
Aminophylline, multiple vitamins, cephalothin Na, pentobarbital, sodium iodide, heparin Na, penicillin G, tetracyclines
Furosemide
8.8-9.3
Acid solutions
Gentamicin sulfate
Do not mix with other medications
Heparin sodium
5-7.5
Sodium bicarbonate, multiple vitamins, tetracyclines, chlorpromazine or promazine, erythromycin lactobionate or gluceptate, gentamicin SO4, hydrocortisone sodium succinate, methylprednisolone Na succinate, kanamycin SO4, meperidine HCl, morphine SO4, penicillin G (K+), protamine
Hydrocortisone sodium succinate
7-8
Cephalothin Na, tetracyclines, heparin Na, kanamycin SO4, multiple vitamins, pentobarbital Na, promazine, erythromycin, tylosin
Kanamycin sulfate
4.5
Cephalothin Na, dextrose, heparin Na, hydrocortisone Na succinate, penicillins, sulfadiazine
Levamisole
Neomycin, phenylbutazone, sulfonamides, tetracyclines
Lidocaine
6-7
Alkaline solutions
Magnesium sulfate
5.5-7
NaHCO3, calcium-containing solutions, tetracyclines
Mannitol
4.5-7
Strongly acidic or alkaline solutions, whole blood, penicillin G
Methylprednisolone sodium succinate
7-8
Aminophylline, heparin Na, tetracyclines, vitamin B
Multiple vitamins
Aminophylline, cyanocobalamin, erythromycin lactobionate, heparin Na, hydrocortisone Na succinate, penicillin G, sodium bicarbonate, tetracyclines
Nitrofurantoin sodium
7.7-9.8
Calcium chloride, insulin, phenol, procaine
Oxytocin
2.5-4.5
Do not mix with other medications
Penicillin G (K+ or Na+)
6-7
Significant inactivation if pH <5.5 or >8
Tetracyclines, acetylcysteine, multiple vitamins, vitamin B with C, lincomycin, aminophylline, pentobarbital, thiopental, cephalothin Na, erythromycin, sodium bicarbonate, sulfonamides, heparin Na, gentamicin
Pentobarbital sodium
10-10.5
Acidic solutions, cephalothin Na, erythromycin, tetracyclines, sodium bicarbonate, succinylcholine chloride
Ringer lactate solution
6-7.5
Epinephrine HCl, tetracyclines, sodium bicarbonate, sulfadiazine Na
Sodium bicarbonate
7-8
Calcium-containing solutions, magnesium sulfate, vitamin B with C, tetracyclines, penicillin G (Na or K), thiopental sodium, pentobarbital Na, streptomycin
Sodium iodide
7.5-9
Vitamin B with C
Succinylcholine chloride
3-4.5
Alkaline solutions, pentobarbital Na, thiopental Na
Sulfonamides
Basic
Acidic solutions, ammonium chloride, gentamicin SO4, kanamycin SO4, lincomycin HCl, methicillin, penicillin G, tetracyclines, vitamin B with C, calcium gluconate, dextrose, tylosin, procaine
Tetracyclines
1.8-2.8
Aminophylline, penicillins, magnesium or calcium salts, cephalothin Na, erythromycin, lactobionate, heparin Na, hydrocortisone Na succinate, methylprednisolone Na succinate, multiple vitamins, nitrofurantoin, novobiocin, polymyxin B, lactated Ringer or Ringer solution, sodium bicarbonate, sulfonamides, barbiturates
Thiamine (vitamin B1)
3-4
Neutral or alkaline pH causes decomposition
Iron salts
Thiopental sodium
10-11
Acidic solutions, promazine HCl, sodium bicarbonate, succinylcholine Cl, atropine SO4, penicillins, cephalosporins, tetracyclines, hydrocortisone Na succinate
Ticarcillin disodium
6-8
Aminoglycosides
Tylosin
Hydrocortisone, tetracycline, streptomycin, sulfonamides
Vitamin B complex
10-11
Alkaline solutions, cephalothin Na, cyanocobalamin, erythromycin lactobionate, hydrocortisone Na succinate, tetracyclines
Vitamin B complex with C
3-6.5
Aminophylline, cephalothin Na, erythromycin lactobionate

Host Factors That Influence Antimicrobial Choice or Dosage
Age
Reproductive Status
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Rational Antimicrobial Therapy
Chapter 45
