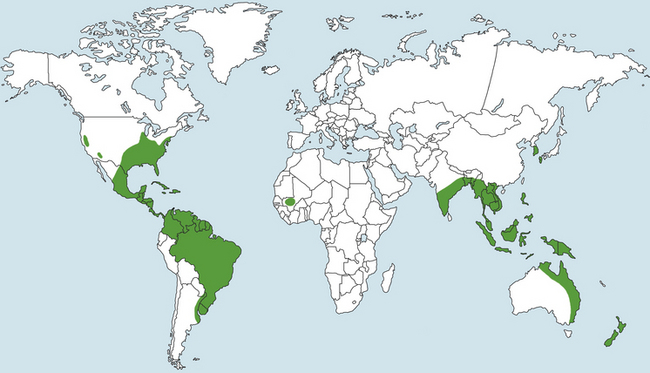
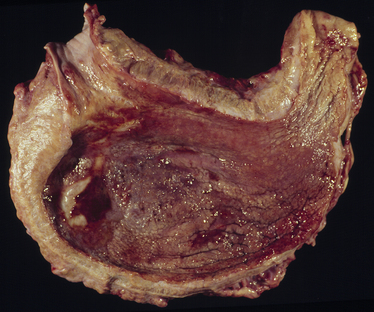
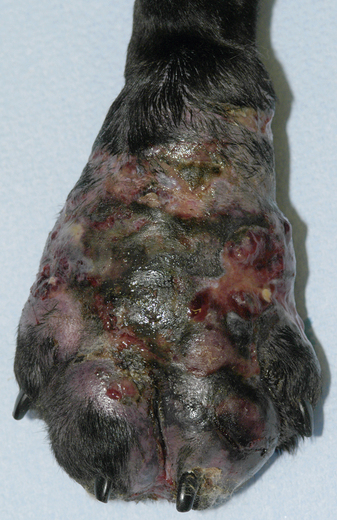
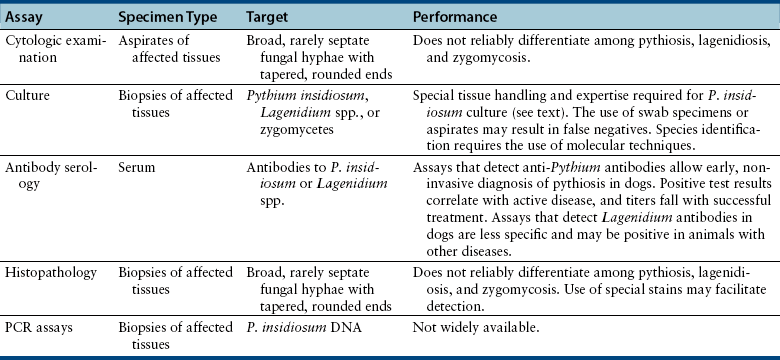
Chapter 69 Pythiosis, lagenidiosis, and zygomycosis are often grouped together because of similarities in their clinical presentations and histologic characteristics (all three cause pyogranulomatous and eosinophilic inflammation associated with large, infrequently septate hyphae with nonparallel walls). Despite their clinicopathologic similarities, however, the pathogens that cause these infections are taxonomically diverse. Pythium insidiosum and Lagenidium species are water molds in the class Oomycetes and as such are more closely related to red algae and Prototheca spp. than to true fungi, including the zygomycetes.1 Important traits that distinguish oomycetes from fungi include the production of motile, flagellate zoospores that act as infective elements in wet environments, and the fact that the oomycete cell membrane generally lacks ergosterol.2 In addition, there are clinically relevant differences in prognosis, recommended treatment, and epidemiology that make it important to distinguish among pythiosis, lagenidiosis, and zygomycosis. Although P. insidiosum has been recognized as a pathogen in dogs and horses for more than 25 years, Lagenidium species have only been recognized as mammalian pathogens since 1999. In comparison to pythiosis and lagenidiosis, infections caused by the zygomycetes are rare. Pythium insidiosum is an aquatic oomycete that causes severe, progressive gastrointestinal or cutaneous disease that is often fatal. The infective form of P. insidiosum is thought to be the motile flagellate zoospore, which is an asexual reproductive structure produced in wet environments in association with plant material.4 Zoospores of P. insidiosum are attracted to damaged tissue, and likely cause infection by encystation on and invasion of damaged skin or gastrointestinal mucosa.5 In support of this mechanism of infection, clinical reports have long suggested an association between pythiosis and frequent exposure to warm freshwater habitats. However, some infections occur in dogs with no history of access to lakes or ponds. Globally, pythiosis occurs in tropical and subtropical climates, including Southeast Asia, eastern coastal Australia, and South America (Figure 69-1). In the United States, the disease occurs most often in the Gulf Coast states, but it has also been recognized throughout the South; along the East Coast as far north as Maryland and New Jersey; in the Midwest including Missouri, Kansas, southern Illinois, and Indiana; and in the West in Arizona and California.6 Pythiosis is most common in young, large-breed dogs, especially Labrador retrievers and other outdoor working breeds. Infected dogs are typically evaluated by veterinarians in the fall, winter, and early spring.7 Pythiosis is uncommon in cats compared to dogs, and specific breed and sex predilections have not been observed in the cases described to date. However, the development of cutaneous pythiosis in very young animals (less than 1 year of age) appears to occur more often in cats than in dogs. For both species, affected animals are typically immunocompetent. The clinical signs associated with pythiosis are caused by gastrointestinal or cutaneous lesions, or by extension of disease into regional lymph nodes or adjacent tissues. Generally, gastrointestinal and cutaneous lesions do not occur together in the same animal. Disseminated pythiosis has only been described in one dog.8 Gastrointestinal pythiosis in dogs is characterized by severe, segmental, transmural thickening of the stomach wall (Figure 69-2), small intestine, colon, rectum, or, rarely, the esophagus, and it is not uncommon to find multiple segmental lesions in the same patient.7,9,10 The most commonly affected regions of the gastrointestinal tract are the gastric outflow area, proximal duodenum, and ileocolic junction. Mesenteric lymphadenopathy is common but is usually caused by reactive hyperplasia rather than infection. Extension of disease into the mesenteric root often causes severe mesenteric lymphadenopathy, with the lymph nodes embedded in a single large, firm mass that is palpable in the cranial to mid abdomen. Invasion of mesenteric vessels may result in bowel ischemia, infarction, perforation, or acute hemoabdomen. In addition, infection may extend into adjacent tissues such as the pancreas or the uterus. Gastrointestinal pythiosis is rare in cats but has been described in two young adult male cats with focal intestinal lesions that were amenable to surgical resection.11 FIGURE 69-2 Gastric pythiosis in a 1-year-old male neutered mixed breed dog. Note extreme thickening of the gastric wall. (Courtesy Amy Grooters, Louisiana State University, Baton Rouge, LA.) Cutaneous pythiosis in dogs occurs most often at the base of the tail, or on the extremities, ventral neck, or perineum,8,12 and is characterized by nonhealing wounds and invasive masses that contain ulcerated nodules and draining tracts (Figure 69-3). In contrast to gastrointestinal pythiosis, regional lymphadenopathy associated with cutaneous pythiosis often reflects extension of infection rather than just reactive hyperplasia. Extension of cutaneous disease to tissues other than regional lymph nodes is rare, but the author has observed a single, focal pulmonary lesion caused by P. insidiosum in one dog with cutaneous pythiosis on a distal extremity. FIGURE 69-3 Cutaneous pythiosis in a 6-month-old female Labrador retriever. (Courtesy Amy Grooters, Louisiana State University, Baton Rouge, LA.) In cats, lesions associated with cutaneous pythiosis include cervical, inguinal, or truncal subcutaneous masses; draining nodular lesions or ulcerated plaque-like lesions on the tailhead or extremities12; and periorbital and nasopharyngeal lesions.13 The most common laboratory abnormalities associated with pythiosis are eosinophilia, anemia, hyperglobulinemia, and hypoalbuminemia. Hypercalcemia has been described once.14 In dogs with gastrointestinal pythiosis, common radiographic findings include poor abdominal detail and the presence of an abdominal mass. Evidence of small bowel obstruction may also be observed. In rare cases of esophageal pythiosis, thoracic radiographs reveals increased soft tissue opacity in the region of the esophagus and deviation of the trachea. Abdominal ultrasonography is the tool of choice for imaging animals suspected to have gastrointestinal pythiosis and usually reveals severe segmental thickening of the gastrointestinal tract and mesenteric lymphadenopathy. In addition, Doppler ultrasonography may identify the presence of vascular pathology (such as an aneurysm) that can result from invasion of mesenteric vessels. In the author’s practice, abdominal ultrasonography is a critical tool for determining the location and extent of disease in dogs with gastrointestinal pythiosis to determine the likelihood that surgical resection could be performed successfully and to assess prognosis. In addition, ultrasonography facilitates the acquisition of fine-needle aspirate specimens from affected segments of the gastrointestinal tract and enlarged lymph nodes. In animals with cutaneous pythiosis, computed tomography may provide information about the extent of disease and may assist with surgical planning when lesions are not located on an extremity.15 Diagnostic assays currently available for pythiosis, lagenidiosis, and zygomycosis in dogs and cats are described in Table 69-1. TABLE 69-1 Diagnostic Assays Currently Available for Pythiosis, Lagenidiosis, and Zygomycosis in Dogs and Cats Cytologically, pythiosis is characterized by pyogranulomatous and eosinophilic inflammation that can be visualized on impression smears made from draining skin lesions or fine-needle aspirates of a thickened gastrointestinal wall or enlarged lymph nodes. Hyphae are observed occasionally in cytologic samples, and their morphologic appearance (broad, rarely septate with tapered, rounded ends) in conjunction with a typical inflammatory response can provide a tentative diagnosis of pythiosis, lagenidiosis, or zygomycosis.14 Isolation of P. insidiosum from infected tissues is not difficult but does require specific specimen handling and culture techniques. For best results, unrefrigerated tissue specimens should be wrapped in a saline-moistened gauze sponge and shipped at ambient temperature to arrive within 24 hours at a laboratory that has experience with culture of pathogenic oomycetes. Small pieces of fresh, nonmacerated tissue are placed directly on the surface of vegetable extract agar supplemented with streptomycin and ampicillin (or an alternative selective medium) and incubated at 37°C.16 Mycelial growth is typically observed within 12 to 24 hours. Isolation of P. insidiosum from swabs of exudate collected from draining skin lesions is generally unsuccessful. Because generation of the sexual reproductive structures that are necessary for definitive morphologic classification of pathogenic oomycetes rarely occurs in the laboratory, identification of P. insidiosum isolates is based on species-specific PCR amplification17 or rRNA gene sequencing. Although production of zoospores is an important supporting feature for the identification of pathogenic oomycetes, it is not specific for P. insidiosum. A species-specific PCR assay has been used to identify P. insidiosum DNA in fresh, frozen, or paraffin-embedded tissues, as well as DNA extracted directly from cultured isolates.18 However, such assays are not widely available on a commercial basis. A highly sensitive and specific ELISA for the detection of anti–P. insidiosum antibodies in dogs is currently available through the author’s laboratory at Louisiana State University.19 This assay provides a means for early, noninvasive diagnosis and also allows response to therapy to be monitored. Following complete surgical resection of infected tissues, a dramatic decrease in antibody levels is typically detected within 2 to 3 months. In contrast, antibody levels remain high in animals that go on to develop clinical recurrence following surgical treatment. This same ELISA has been adapted for detection of anti–P. insidiosum antibodies in domestic and exotic cats, and although case numbers are too small to generate strong estimates of sensitivity and specificity, in the author’s experience they appear to be high. Histologically, pythiosis is characterized by eosinophilic pyogranulomatous inflammation, with multiple foci of necrosis that are surrounded and infiltrated by neutrophils, eosinophils, and macrophages. In addition, discrete granulomas composed of epithelioid macrophages, plasma cells, multinucleate giant cells, and fewer neutrophils and eosinophils are often observed. Organisms are typically found within areas of necrosis or at the center of granulomas. Vasculitis is occasionally present. In gastrointestinal pythiosis, inflammation centers on the submucosal and muscular layers rather than the mucosa and lamina propria. Therefore, the diagnosis of pythiosis may be missed if endoscopic biopsies that fail to reach deeper tissues are submitted. Similarly, disease in animals with cutaneous pythiosis is typically found in the deep dermis and subcutis, necessitating deep wedge biopsies rather than punch biopsies for optimal evaluation. Pythium insidiosum hyphae are not routinely visualized on H&E-stained sections but may be identified as clear spaces surrounded by a narrow band of eosinophilic material. Hyphae are readily visualized in sections stained with Gomori’s methenamine silver (GMS) but usually do not stain well with periodic acid–Schiff (PAS). They are wide (mean, 4 µm; range, 2 to 7 µm), have nonparallel walls, are infrequently septate, and occasionally branch at right angles.7,8 Aggressive surgical resection of all infected tissues with wide margins is the treatment of choice for pythiosis. In animals with cutaneous lesions that are confined to a single distal extremity, amputation should be recommended unless there is evidence of regional lymph node infection. In patients with gastrointestinal pythiosis, segmental lesions should be resected with 5-cm margins whenever possible. Because mesenteric lymph nodes are usually reactive rather than infected, the presence of nonresectable mesenteric lymphadenopathy should not dissuade the surgeon from pursuing resection of a segmental bowel lesion. Enlarged regional lymph nodes should, however, always be biopsied for prognostic information. In animals with cutaneous pythiosis, the surgeon should attempt to obtain skin margins of 5 cm and deep margins of two fascial planes.15 Enlarged regional lymph nodes in dogs with cutaneous pythiosis are often infected rather than reactive; therefore, they should be evaluated cytologically or histologically before amputation or other aggressive resection is attempted. Unfortunately, many dogs with pythiosis are not brought to a veterinarian until late in the course of disease, when complete excision is not possible. In addition, the anatomic location of the lesion may prevent complete surgical excision when gastrointestinal pythiosis involves the esophagus, gastric outflow tract, rectum, or mesenteric root, or when cutaneous pythiosis involves the ventral thorax, base of the tail, or trunk. Still, very aggressive surgery such as a partial gastrectomy14 or massive resection of cutaneous lesions combined with reconstructive techniques15 can sometimes result in a successful outcome. Local postoperative recurrence of pythiosis is common (especially when wide surgical margins cannot be achieved) and can occur either at the site of resection or in regional lymph nodes. For this reason, postoperative medical therapy is often recommended when the surgeon is not confident that margins of at least 5 cm were achieved. In the author’s practice, dogs with focal midjejunal lesions that are resected with wide margins and dogs without regional lymphadenopathy that undergo amputation for distal extremity lesions are not routinely treated with postoperative antifungal medication. All other patients with pythiosis typically are treated with itraconazole (10 mg/kg PO q24h) and terbinafine (5 to 10 mg/kg PO q24h) for at least 2 to 3 months after surgical resection. Unfortunately, if resection is incomplete, lesions often progress despite medical therapy, and clinical signs recur within weeks to months. Medical therapy for nonresectable pythiosis is typically unrewarding, likely because ergosterol (the target for most currently available antifungal drugs) is generally lacking in the oomycete cell membrane. Despite this fact, clinical and serologic cures occur in some patients treated with a combination of itraconazole (10 mg/kg PO q24h) and terbinafine (5 to 10 mg/kg PO q24h).20 Although the percentage of animals that respond to this treatment is still low, the combination protocol anecdotally appears to be superior to itraconazole or amphotericin B alone. An immunotherapy product derived from antigens of P. insidiosum has been used successfully to treat pythiosis in horses and people.21,22 Unfortunately, although controlled trials have not been completed, the efficacy of this product in dogs appears to be poor, and clinical improvement has not been observed in any of the author’s patients.
Pythiosis, Lagenidiosis, and Zygomycosis
Pythiosis
Clinical Features
Gastrointestinal Pythiosis
Cutaneous Pythiosis
Diagnosis
Imaging Findings
Microbiologic Tests
Cytologic Examination
Culture
Molecular Diagnosis using the Polymerase Chain Reaction
Serologic Testing
Pathologic Findings
Treatment and Prognosis
Pythiosis, Lagenidiosis, and Zygomycosis
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree