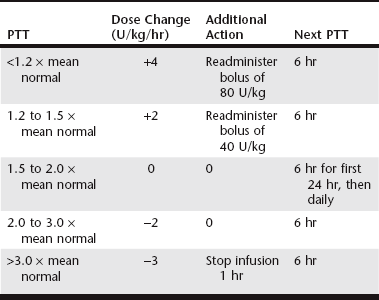
Chapter 166 The term hypercoagulable state refers to disorders in patients with underlying disease known to be associated with thrombophilia and an increased risk of thrombosis. In animals, these are acquired disorders, and the pathogenesis is generally multifactorial and complex. PTE in dogs is associated with IMHA, protein-losing nephropathy (PLN), neoplasia, necrotizing pancreatitis, hypercortisolism (hyperadrenocorticism and corticosteroid therapy), protein-losing enteropathy (PLE), cardiac disease (dirofilariasis, endocarditis, and cardiomyopathy), diabetes mellitus, sepsis, atherosclerosis, trauma, and major surgical procedures. In canine necropsy studies, 59% and 64% of dogs with PTE had more than one disorder potentially causing hypercoagulability, and many of these dogs also had thrombosis in nonpulmonary organs (LaRue et al, 1990; Johnson et al, 1999). PTE in cats is associated most commonly with neoplasia or cardiomyopathy; other infrequently reported conditions include necrotizing pancreatitis, IMHA, PLN, PLE, hypercortisolism, and sepsis. In one study, 47% of cats had multiple potentially prothrombotic disease processes (Norris et al, 1999). The anticoagulant effects of a standard dose of UFH vary widely among patients. Bioavailability after subcutaneous administration is variable and often poor. The plasma clearance of UFH depends on a rapid, dose-related, saturable cellular mechanism and a slower, non–dose-related renal clearance. For this reason, the intensity and duration of effect increase disproportionately with increasing dose. Higher-molecular-weight species are cleared more rapidly than lower-molecular-weight species, which results in varied anticoagulant activity over time. Binding of UFH to plasma proteins, endothelial cells, and platelets contributes to the unpredictable response. Moreover, the concentration of UFH-binding proteins is increased in patients with inflammation, which results in heparin resistance and necessitates the use of higher doses to achieve effective anticoagulation. Because of the markedly variable pharmacokinetic profile of UFH, successful therapy requires monitoring of the anticoagulant response and titration of the dose to the individual patient (Hirsh and Raschke, 2004). It has been shown in human patients that, in the absence of such measures, many patients receive inadequate heparinization, which results in an increased incidence of recurrent thromboembolism. Anticoagulant response to UFH traditionally is monitored using the partial thromboplastin time (PTT) because point-of-care testing enables practical dose titration. The therapeutic goal in human patients is a PTT of 1.5 to 2.5 times control or pretreatment values. Studies suggest that this goal results in supratherapeutic heparin concentrations in dogs, and a target PTT range of 1.5 to 2.0 times baseline has been suggested. A limitation in the use of the PTT to guide UFH therapy is that this measure is not correlated directly with anticoagulant activity and clinical efficacy, largely because the effect of UFH on PTT reflects primarily its factor IIa inhibition. A more accurate method for monitoring UFH effects is measurement of plasma anti-Xa activity, which has been correlated directly with plasma heparin concentration. However, results of this test are not commonly available for same-day dosage adjustments. It is unclear whether thromboelastography (TEG) will prove useful for guiding UFH therapy. A study in healthy dogs implied that TEG parameters are affected by even low plasma levels of heparin, and thus the test may be too responsive to identify adequate or excessive anticoagulation (Pittman et al, 2010; see Chapter 15). In human patients, intravenous continuous-rate infusion of UFH using a heparin nomogram has been the traditional standard of care for acute PTE (Hyers et al, 2001). The author uses an extrapolation of this nomogram in dogs (Table 166-1). There are no reports of such an application in cats. Subcutaneous UFH is not recommended for acute therapy because this route is unreliable in achieving therapeutic ranges rapidly. Even high dosages of UFH (300 U/kg q6h SC) and titration based on PTT or anti-Xa activity failed to result in therapeutic ranges within 48 hours in 10 of 18 dogs with IMHA (Breuhl et al, 2009). Therefore SC UFH should not be used in patients with IMHA suspected of having PTE unless other means of therapy are not feasible. In cats, UFH at a dosage of 250 U/kg q8h SC commonly is recommended as part of the initial treatment regimen for arterial thromboembolism. A small study in healthy cats demonstrated anti-Xa activity within or above the target range at this dosage, but correlation with PTT results currently is unclear (Alwood et al, 2007). Limited data are available regarding dosing protocols in dogs and cats, and the clinical efficacy or superiority (compared to UFH) of the LMWHs is not yet established. A number of studies in healthy animals have evaluated the pharmacokinetics of dalteparin (Fragmin) and enoxaparin (Lovenox), primarily with respect to anti-Xa activity. In canine studies, dalteparin administered at 150 U/kg SC achieved therapeutic ranges in healthy dogs (Mischke et al, 2001). Pharmacokinetic studies of dalteparin in cats show excellent bioavailability, with peak levels within 2 hours of administration (Alwood et al, 2007). Dalteparin at 100 U/kg SC did not result reliably in peak therapeutic anti-Xa activity, whereas a dose of 200 U/kg was supratherapeutic in some cats. Therefore a dose of 150 U/kg has been recommended, although it has not been investigated fully. A small study in healthy greyhound dogs indicated that enoxaparin at 0.8 mg/kg SC achieved target anti-Xa levels within 4 hours of administration (Lunsford et al, 2009). Enoxaparin at 1.0 mg/kg has been shown to result in appropriate anticoagulation in experiments in cats (Van De Wiele et al, 2010). The preceding studies showed waning of anti-Xa activity below therapeutic range within 6 to 8 hours. As a result, investigators have recommended more frequent dosing intervals in dogs and cats (every 6 to 8 hours). However, these recommendations are controversial. The therapeutic target in human patients is peak anti-Xa activity of 0.5 to 1.0 U/ml measured at 4 hours; however, it is unlikely that this activity must be maintained, and the minimal effective anti-Xa activity in humans is undetermined. Also, in the feline venous stasis model of Van De Wiele and colleagues (2010), enoxaparin at 1 mg/kg q12h reliably resulted in a measurable antithrombotic effect that persisted well after anti-Xa levels had declined below target range. It is also worth noting that the applicability in other species of therapeutic anti-Xa activity established in humans has not been verified. This complexity underscores the need for clinical trials with outcome measures.
Pulmonary Thromboembolism
Treatment of Pulmonary Thromboembolism
Secondary Thromboprophylaxis
Unfractionated Heparin
Low-Molecular-Weight Heparin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pulmonary Thromboembolism
Only gold members can continue reading. Log In or Register to continue