J. Jill Heatley, Juan Cornejo Psittaciformes is a homogeneous order of over 350 extant species of parrots grouped in about 84 genera.12 The three superfamilies consist of Strigopoidea, which includes the kakapo (Strigops habroptilus), the kea and the kaka (Nestor spp.); Cacatuoidea, which includes the black and white cockatoos (Cacatuoidea, Calyptorhynchinae, and Cacatuinae), and the cockatiel (Nymphicinae, 1 sp.); and Psittacoidea, which comprises the remaining 326 species.26,59 Parrots are found mainly in the tropical and subtropical regions of the Southern Hemisphere, with the greatest diversity in the New World and Australia. Most parrots are diurnal and arboreal. Common habitats include moist forest, woodland, and savanna; few species prefer open areas. Their bright colors, mimicry ability, and charisma have made parrots popular in captivity for centuries. In part because of this popularity and in part because of loss or degradation of their habitat, parrots are the most endangered birds in the world. At least nine species have become extinct since 1600, over 25% of the extant species are listed as threatened, and an additional 11% are listed as near threatened.25 The most remarkable anatomic characteristic of the parrots is their broad, hooked bill and the complex associated musculature. The upper mandible is prominent and down-curved and fits over the broad shorter up-curved lower mandible. The upper mandible articulates with the skull, allowing extensive movement of both mandibles and an increased biting pressure. Most Psittaciformes species have a thick, muscular tongue, which, when used in combination with the bill, is an effective tool to manipulate and process food. In the subfamily Loriinae (lories and lorikeets) the tongue tip is further modified with erectile dermal papillae for gathering nectar and pollen. Parrots have short tarsi and zygodactyl (yoke-toed) feet, and the first (hallux) and fourth toes orient posteriorly, as an adaptation for climbing trees and manipulation of objects. The head is proportionally large and broad, the neck is short, and the ceca are vestigial or absent. Most species have brightly colored plumage, and only a few species have sexual dimorphism, apparent to the human eye. Juvenile parrots often have slightly dull plumage and a darker iris compared to adults. Parrots vary widely in size, from the Hyacinth Macaw (Anodorhynchis hyacinthinus) reaching 100 centimeters (cm) to the flightless Kakapo weighing up to 3 kilograms (kg) to the 8-cm 10-gram (g) pygmy parrots (Micropsitta spp.). The superfamily Cacatuoidea is distinguished by the presence of a gall bladder, the superficial position of the left carotid artery, the ossified orbital ring in the skull, the absence of blue and green plumage colors, and the presence of a movable feathered crest.2,38,53 Because of their powerful beaks and propensity to chew, parrots are best housed in metal enclosures. Galvanized box wire mesh of 1 × 1 inch is an option for many species; however, some of the larger macaws and cockatoos require more secure welds, and may be able to navigate complex locking systems. New galvanized wire should be washed with a 1 : 10 vinegar solution and rinsed with water to remove the deposits of zinc that are likely to be toxic. Young or small birds with bills that fit into mesh of this size may be more at risk of toxicity when this type of enclosure is used. Except in large aviaries, it is difficult to maintain live plants in parrot enclosures. A soft substrate such as bark chips, sand, or soil is best, as it can be easily cleaned and dried to avoid fungal overgrowth. Suspended cages that limit access to feces or discarded food are a popular option for housing breeding pairs. Most parrots are not cold hardy and should not be kept below 50° F (10° C) without supplemental heat. Acclimatization may facilitate the parrot to tolerance of approximately 30° F (0° C) for short periods, with protection from the elements during inclement weather. Daily access to fresh air and sunlight are highly recommended for the health and well-being of these species and to promote good bone density and feather quality. To fulfill their enrichment and chewing needs, parrots should also be provided with a regular supply of fresh branches to minimize damage to the perches and live plants in their enclosures. See Table 21-1 for breeding parameters of common species in North American zoos. TABLE 21-1 Characteristics of Common Psittaciforme Species Kept in North American Zoos (as of 31 December, 2011)* * Suggested outdoor aviary, nest box, and leg band are based on author experience (JC). In the wild, most parrots consume a variety of plant-based diets (seeds, fruits, buds, bark, roots, flowers, nectar) that include occasional insects and are generally classified as herbivores.29 Field conditions make determination of food sources and quantification of food consumption challenging. Thus, little information on the nutritional content of wild adult parrot diets exists3,14 despite an increasing volume of research on the nutrition of captive Psittaciformes.8,11,16,22,28,30–33,44,45,50,57,58,61 Because of a lack of complete understanding of nutritional requirements for growth and maintenance in captive parrots, malnutrition is still one of the main concerns in the care and propagation of this group, and providing nutritionally adequate diets remains a serious concern.21,23,31,43,48 Parrot diet formulation must account for caloric density, as this determines how much food the bird will eat and, thus, the amount of each nutrient consumed.29 For this reason, diets of free-ranging parrots will tend to be deficient if extrapolated to captive birds. Most recommended diets for Psittaciformes are based on studies on poultry modified by research for small granivorous species (budgerigars, Melopsittacus undulatus, and cockatiels, Nymphicus hollandicus); therefore, it is unlikely that they adequately model all the dietary requirements of the diverse members of the order. Traditional diets of captive parrots have been seed based. Most commercial seeds are high in fat; have a low calcium-to-phosphorus (Ca : P) ratio; and low levels of Ca, P, sodium (Na), zinc (Zn), iron (Fe), lysine, and vitamin A, based on metabolic energy needs.24,56 Thus, deficiencies of these nutrients are common in captive parrots.54,56 The addition of fruits and vegetables to a seed mix will not always result in a complete and balanced diet, as parrots will preferentially consume high-energy seeds.4,10,24,54 Formulated processed diets available from many manufacturers provide the best available option for complete nutrition. To fulfill parrots’ environmental enrichment needs, these diets may have up to 25% fresh low-energy-density vegetables and fruits added and still be within recommended dietary ranges.4,25,55 Lories and lorikeets (Loriini) have unique anatomic adaptations to feed on nectar and pollen, and their diet in captivity should ideally be liquid.13,60 Several nectar products are commercially available to provide parrots with complete nutrition, and fruits and vegetables may be added to provide diversity and enrichment. Capture of parrots in large enclosures may be challenging. Training birds to station, target, load into carriers, or present at the side of the cage is recommended to facilitate examination and preventive medical procedures. Otherwise parrots may be netted or restrained initially with a towel. Nets with mesh size smaller than the bird’s head and feet to lessen the risk of entanglement, a net hoop that is wide enough to cover the entire animal, and a net with fabric that is long enough to allow the bird to be secured into the bottom by folding over (locking) of the net should be chosen. Once the birds are captured, two handlers are required to extract large parrots from the net while avoiding the claws and bill. Physical restraint and handling are acceptable in most parrot species. Even the largest of parrot species may be adequately restrained by two people with appropriate handling skills. Correct methods for handling Psittaciformes include aspects that limit the likelihood of damage to the handler, the examiner, and the patient. For handler protection, the patient should be held in such a way as to not be injured by the bill and the claws. The keel should be allowed to move freely for respiration, the wings held to the side of the body to avoid damage to the appendages, and the feet adequately restrained to allow easy examination. Generally, a complete ring made with the thumb and the forefinger and placed below the mandible allows for neck extension and good restraint while allowing excellent airflow to the bird’s respiratory system. No attempt should be made to restrain parrots by gripping the lateral aspects of the mandible, as the delicate bones in this area as well as the tissues of the bare facial patch on some parrots may be seriously damaged. The bill, which has approximately 400 pounds or greater of bite force in some species, cannot be adequately restrained by using this method. Insertion of the thumb or other digit into the gular area beneath the mandible of the beak for extension of the head is also not recommended, as the glottis may be, inadvertently, obstructed in this manner. In small parrots, the entire body may be cupped in the palm of the hand, while the head, held between the index and middle finger, is extended to allow adequate restraint. The bird’s body should always be supported. The overlapping signet ring formation of the trachea allows fairly firm restraint in this manner without risk of tracheal collapse. Although some may prefer lightweight leather gloves for field work with parrots, these provide little protection from the crushing force of the bill. Similarly, a towel only provides a “hide” or foil for the holders’ hands, much like the cape of a matador. Ear plugs are also recommended if the parrot is anticipated to be so loud that the staff must raise their voices to communicate during examination to avoid damage to the human auditory system; even parrots that are quite small, for example, the sun conure (Aratinga solstitialis), may emanate sound meant to be heard over long distances. Much work has been accomplished lately in these intelligent species on positive reinforcement training for a variety of medical examinations and procedures. Birds have been trained to target, station, load into carriers, and accept medications from syringes and intramuscular injections. Whenever possible, these techniques should be incorporated into the daily care and enrichment of Psittaciformes to reduce stress and increase veterinary ability to provide medical care without undue stress to the patient. Sedation of Psittaciformes species with midazolam and butorphanol, which may be given intranasally (IN) or intramuscularly (IM), has become popular for use with companion parrots and has also been used in the zoo setting.37 Based on the tolerance of different species to these drugs, it is advisable to begin with a low dose and to have reversal drugs available. Additional medications used for pain control in these species include meloxicam, carprofen, and tramadol. For anesthesia, induction with sevoflurane or isoflurane via a face mask is most commonly used; Generally, parrots are not intubated for short-term anesthesia (<20 minutes); an uncuffed endotracheal tube is used if intubation is necessary. Fasting to ensure an empty crop is recommended prior to anesthesia induction. Parrots may also be intubated through other air sacs when access to the oral cavity, bill, or trachea is desired for surgery. Excellent reviews of avian anesthesia, applicable to parrots, may be found elsewhere.19 Common surgical procedures include endoscopy for internal examination, gender determination, and biopsy. Tumor removal, bill repair or restructuring, amputation or other orthopedic repair, and reproductive system surgery are also common. Surgical approaches should be tempered by constraints of patient positioning to allow free keel mobility and observation. Additionally, surgical preparation and perioperative care should incorporate techniques to avoid excessive heat loss and to provide heat supplementation, for example, minimal feather removal, use of noncooling or warmed liquids, and transparent draping. Flammable liquids as preparatory agents must be avoided when electrosurgical, radiosurgical, or laser surgical techniques are used. Foreign body removal is often an indication for veterinary intervention. However, in parrots, alternatives to exploratory coeliotomy should be considered, as proventriculotomy, ventriculotomy, and enterotomy are not without risk of morbidity and mortality. In many cases, flexible or rigid endoscopy, accompanied by ingluviotomy if necessary, is a reasonable alternative approach for foreign body removal; use of neodymium magnets embedded in the end of flexible tubing for removal of metal objects has also been successful as a less invasive, although blind, method if fluoroscopy is not available. Because of the tough ventricular koilin lining and the very muscular ventriculus, foreign objects that are not obstructive may not need removal, as they will be disintegrated slowly by normal GI action and motility. However, this same action often results in continuing toxic insult and blood accumulation if the object contains lead, zinc, or copper. As neoplasms are not uncommon in these long-lived species, tumor removal should be followed up with histopathologic examination to provide an appropriate standard of care for parrots. Principles for surgical approach, orthopedic repair, and amputation of pelvic and thoracic limbs to be used in parrots have been published.41 Many captive parrots do well with coaptive or conservative management of thoracic and pelvic limb injuries as flight and full talon function are not required for eating and perching. Surgery to limit flight is not recommended in these species because of their longevity and predisposition to atherosclerosis and obesity. Surgery or repair of the bill is challenging because of the numerous functional joints and fragile bones in this area. Computed tomography (CT) is recommended prior to bill surgery to determine the best approach for surgical correction. Correction of bill malformation in these species (scissor bill, mandibular prognathism) is best attempted when the birds are young.2 Many parrots with complete or partial loss or malformation of the upper or lower bill may adapt to feeding and maintain normal body weight and social bonds. Reproductive surgery is a common concern in female parrots which produce too many eggs. Some parrots may lay eggs until total body calcium stores become depleted, resulting in pathologic fractures, tetany, and paresis. Salpingohysterectomy does not guarantee cessation of ovulation, and ovary removal is challenging because of proximity to the adrenal gland, the cranial pole of the kidney, and local vessel anatomy. Hormonal modulation via synthetic gonadotropin-releasing hormone (GnRH) agonists may decrease reproductive organ activity, facilitating surgical intervention or negating the need for surgical intervention. However, environmental factors, species factors, and individual patient factors all affect reproductive activity, so treatment and environment should be modified accordingly for best patient outcome. Surgery for cloacal prolapse is common for Cacatua spp. Although surgical reduction of vent aperture or tacking of the extruded cloaca may reduce the prolapse in the short term, hormonal and inappropriate physical stimulation of the birds and the inappropriate bonding behavior of keepers or owners may result in breakdown of this repair, which is associated with morbidity and mortality.46 Surgery alone will not often repair a behavioral, hormonal, and usually longstanding, problem. Because the cloaca is the nexus of three different systems, cloacal prolapse may involve not only the GI tract but also the urinary and reproductive tracts. Care should be taken to discern which aspects of these systems are prolapsed prior to attempting surgical repair and to counsel the primary caretaker to correct any behavioral problems prior to attempting this non-emergency surgery. Numerous pharmaceuticals are of use in Psittaciformes. Pharmacologic concerns in parrots include regurgitation (in macaws) after administration of trimethoprim sulfa and upon recovery from isoflurane or sevoflurane anesthesia. Birds should be allowed to regurgitate without restraint to avoid aspiration. Doxycycline, a drug commonly used in Psittaciformes to treat chlamydial infection, is an effective chelator; however, administration of supplemental calcium should be considered in young growing birds or possibly in hypocalcemic hens when using this drug. Administration of long-acting topical steroids such as triamcinolone, commonly available for use in companion mammals, or long term administration of systemic steroids are not recommended for use in parrots, as they may cause overwhelming fungal infection and mortality. Although pharmacotherapy is often used in an attempt to control feather-destructive behavior, a full diagnostic workup is necessary to rule out medical causes and to assess baseline health status prior to administration of behavioral modification drugs. In-depth assessment of the enrichment needs of the parrot patient, prior to drug administration, is also highly recommended. Physical examination of parrots should progress in a manner similar to that in other species. However, observational examination should proceed prior to physical examination to avoid unexpected patient death resulting from physical restraint. Parrots often mask signs of illness until decompensation is near total and mortality is impending. Therefore, when severe clinical signs of illness are apparent on observational examination, full physical examination may be forgone in the critical patient to preserve life via critical supportive therapies such as provision of warmth, oxygen, and fluids; administration of antibiotics; and pain control. After patient stabilization, a more complete physical examination may be performed on the live parrot. Physical examination should progress in a timely manner, and sometimes a stepwise physical examination with periods of rest for the patient may be indicated. Based on observational examination of the patient, if the neurologic, respiratory, and musculoskeletal systems of the patient appear to be in order, physical examination generally proceeds from head to toe as with companion mammals. Special attention should be given to examination of the choanal papilla; symmetry; body condition; auscultation of the lungs, air sacs, and heart; palpation of the pulse and assessment of venous refill time on the wing; hydration; and the ventrum of the feet. Examine the vent for mucous membrane roughening associated with papillomas. Grip and strength of the bill and feet and the withdrawal response of the limbs, as well as joint mobility, should be assessed. Coelomic palpation in most parrots will reveal a concave conformation, and the ventriculus is often palpable. The jugular apteria and ears should also be assessed, as trauma is often obscured until the feathers are parted to observe the transparent skin and subcutaneous tissues. Feathers should be observed for stress lines, fractures, or color changes, and the bill should be assessed for weakness or overgrowth. The uropygial gland, present in most parrots but absent in Amazona and Anodorhynchus spp., should be visualized and palpated. Common infectious diseases of concern are listed in Tables 21-2, 21-3, and 21-4. Few bacterial diseases of parrots are transmissible to man, but infection with Chlamydia spp. is the most important exception. Clostridial infection, pasteurellosis, and multiple gram-negative bacterial infections are of most immediate concern for parrot health, as they may lead to rapid sepsis and acute death. Mycobacterial disease is not uncommon in parrot species; zoonotic potential, challenging assessment and diagnosis, and the necessary prolonged multidrug therapy often result in euthanasia of these patients. Salmonella spp. as well as methicillin-resistant Staphylococcus aureus and other multidrug-resistant bacteria may be carried by parrots, and although morbidity is likely in the avian patient, concern with regard to infection is primarily based on zoonotic potential. Numerous bacteria with possible human pathogenicity have now been cultured from apparently healthy captive and free-living parrots.62 Similarly, normal flora of humans may pose some risk to the health of parrots.6 Bacterial diseases of particular significance to both parrot and human health are listed in Table 21-2. TABLE 21-2 Selected Bacterial Diseases of Psittaciformes
Psittaciformes
General Biology
Unique Anatomy
Special Housing Requirements
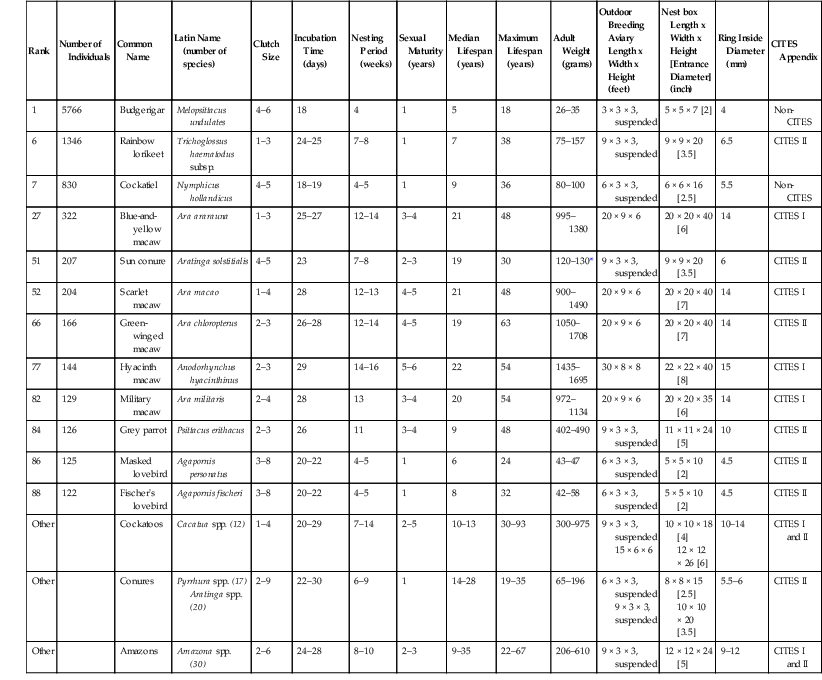
Rank
Number of Individuals
Common Name
Latin Name (number of species)
Clutch Size
Incubation Time (days)
Nesting Period (weeks)
Sexual Maturity (years)
Median Lifespan (years)
Maximum Lifespan (years)
Adult Weight (grams)
Outdoor Breeding Aviary Length x Width x Height (feet)
Nest box Length x Width x Height [Entrance Diameter] (inch)
Ring Inside Diameter (mm)
CITES Appendix
1
5766
Budgerigar
Melopsittacus undulates
4–6
18
4
1
5
18
26–35
3 × 3 × 3, suspended
5 × 5 × 7 [2]
4
Non-CITES
6
1346
Rainbow lorikeet
Trichoglossus haematodus subsp.
1–3
24–25
7–8
1
7
38
75–157
9 × 3 × 3, suspended
9 × 9 × 20 [3.5]
6.5
CITES II
7
830
Cockatiel
Nymphicus hollandicus
4–5
18–19
4–5
1
9
36
80–100
6 × 3 × 3, suspended
6 × 6 × 16 [2.5]
5.5
Non-CITES
27
322
Blue-and-yellow macaw
Ara ararauna
1–3
25–27
12–14
3–4
21
48
995–1380
20 × 9 × 6
20 × 20 × 40 [6]
14
CITES I
51
207
Sun conure
Aratinga solstitialis
4–5
23
7–8
2–3
19
30
120–130*
9 × 3 × 3, suspended
9 × 9 × 20 [3.5]
6
CITES II
52
204
Scarlet macaw
Ara macao
1–4
28
12–13
4–5
21
48
900–1490
20 × 9 × 6
20 × 20 × 40 [7]
14
CITES I
66
166
Green-winged macaw
Ara chloropterus
2–3
26–28
12–14
4–5
19
63
1050–1708
20 × 9 × 6
20 × 20 × 40 [7]
14
CITES II
77
144
Hyacinth macaw
Anodorhynchus hyacinthinus
2–3
29
14–16
5–6
22
54
1435–1695
30 × 8 × 8
22 × 22 × 40 [8]
15
CITES I
82
129
Military macaw
Ara militaris
2–4
28
13
3–4
20
54
972–1134
20 × 9 × 6
20 × 20 × 35 [6]
14
CITES I
84
126
Grey parrot
Psittacus erithacus
2–3
26
11
3–4
9
48
402–490
9 × 3 × 3, suspended
11 × 11 × 24 [5]
10
CITES II
86
125
Masked lovebird
Agapornis personatus
3–8
20–22
4–5
1
6
24
43–47
6 × 3 × 3, suspended
5 × 5 × 10 [2]
4.5
CITES II
88
122
Fischer’s lovebird
Agapornis fischeri
3–8
20–22
4–5
1
8
32
42–58
6 × 3 × 3, suspended
5 × 5 × 10 [2]
4.5
CITES II
Other
Cockatoos
Cacatua spp. (12)
1–4
20–29
7–14
2–5
10–13
30–93
300–975
9 × 3 × 3, suspended
15 × 6 × 6
10 × 10 × 18 [4]
12 × 12 × 26 [6]
10–14
CITES I and II
Other
Conures
Pyrrhura spp. (17)
Aratinga spp. (20)
2–9
22–30
6–9
1
14–28
19–35
65–196
6 × 3 × 3, suspended
9 × 3 × 3, suspended
8 × 8 × 15 [2.5]
10 × 10 × 20 [3.5]
5.5–6
CITES II
Other
Amazons
Amazona spp. (30)
2–6
24–28
8–10
2–3
9–35
22–67
206–610
9 × 3 × 3, suspended
12 × 12 × 24 [5]
9–12
CITES I and II
Feeding
Restraint and Handling
Surgery (Common and Special Considerations)
Other Pharmaceuticals
Physical Examination and Diagnostics
Disease
Bacterial Disease
Etiology
Epizootiology
Signs
Diagnosis
Management
Chlamydiosis, ornithosis, parrot fever, psittacosis
Chlamydia psittaci
Obligate intracellular bacteria, gram negative.
Life cycle consists of the infectious elementary body, which may survive outside the host and infects the host epithelial cells, becoming the reticulate body, which exists inside the host and reproduces by binary fission. Only the elementary body is immunogenic, as the reticulate body evades the host immune system inside the cell.
All parrots are susceptible and disease is circumglobal.
Parrots seem particularly suited to harbor this pathogen.
Incubation ranges from 3 days to 2 months.
Duration of immunity is negligible after infection and treated birds may become reinfected. Antibodies are not protective.
Parrots may harbor C. psittaci without clinical signs.
When present, clinical signs are often nonspecific, as the organism affects the gastrointestinal tract, liver, and the respiratory and neurologic systems. Birds often appear depressed and lethargic and may have bright green stools because of liver disease. Disease may be limited to the upper respiratory system or conjunctivitis.
Clinical signs may mimic heavy metal intoxication.
Chlamydiosis is often a diagnostic challenge in the live bird. Isolation of the organism for definitive diagnosis requires specialized media and is rarely pursued based on expense.
Elementary body agglutination was the preferred test for diagnosis of active infection, especially when combined with an immunoglobulin M (IgM) titer; however, this test is no longer commercially available.
Most commonly, a choanal or cloacal swab is submitted for polymerase chain reaction (PCR) testing to determine if the organism is being shed. The presence of the organism, consistent clinical signs, and severe leukocytosis are often combined to make a diagnosis consistent with suspicion of chlamydiosis.
PCR and detection of intracytoplasmic chlamydial inclusions often found in the enlarged liver or spleen at necropsy are diagnostic. Special stains or indirect fluorescent antibody (IFA) testing are helpful for detection of these inclusions, which are rare in oral, ocular or respiratory secretions or conjunctival scrapings.
This zoonotic organism may cause mild to severe respiratory disease in humans with flu-like symptoms. However, disease is rare in humans with less than 50 cases confirmed since 1996. Treatment in humans and birds is prolonged (at least 45 days) because of the occult and protracted lifecycle of C. psittaci. Tetracyclines, doxycycline, azithromycin, or fluoroquinolones may be used for treatment.
This organism and the required repeated testing are the primary reasons for Psittaciformes to be quarantined and carefully screened prior to entry into a zoologic collection for 45 to 60 days. Required treatment and additional testing in cases of positive birds may prolong required isolation.
No vaccine is available.
Elementary bodies are environmentally stable but may be inactivated with ultraviolet (UV) light, 70% ethanol, quaternary ammonium, or 3% hydrogen peroxide.
Clostridium
Clostridium perfringens
Gram-positive, rod-shaped, anaerobic, spore-forming bacterium that is ubiquitous in nature and a common component of decaying vegetation.
Ingestion of clostridial organisms generally occurs because of spoiled high-sugar (nectar or fruit) food stuffs. Spoilage may occur within hours in hot weather.
Some parrots such as the Kakapo may have clostridial organisms normally within their gastrointestinal (GI) tracts.55
Birds often die acutely without clinical signs. Parrots that survive longer may show lethargy, depression, and dehydration; foamy malodourous droppings caused by gas production of some organisms may be observed. Observed clinical signs prior to death are the exception rather than the rule and usually limited to parrots that are not nectivores.
Antemortem diagnostics are often limited in these cases because of the extremely unstable nature of the patient. Radiography and fecal cytology may provide supportive evidence of spore-forming, gas-producing bacterial infection. The bird may be in poor condition or more often very good body condition at necropsy with minimal gross signs. Diagnosis is often made after death histologically. Necrosis of the GI mucosa and colonization of villi by large gram-positive rods consistent with Clostridium spp. are found.
C. perfringens is a common cause of foodborne illness in humans but has minimal zoonotic potential, as it occurs in aviaries.
Supportive care should include hydration, antibiotic treatment with appropriate spectrum, and antiendotoxin treatment. Prognosis in all but the most stable cases, where findings may be incidental, is guarded.
Appropriate disinfection of food containers and utensils should occur daily if not more often to maintain cleanliness, and sugar-containing food should be removed
and replaced as often as every 4 hours in hot weather, which is conducive of growth of these organisms. Facilities which cannot adhere to these standards or have continuing outbreaks should consider limiting nectivores in their collections. C. perfringens enterotoxin (CPE), which mediates disease, is inactivated at 74° C (165° F)
Colibacillosis
Coligranuloma
Escherichia coli
Gram-negative, facultative anaerobic, rod-shaped bacterium, common in the lower intestine of many mammals.
All parrots considered susceptible; but reported in the Amazon parrot, Hyacinth macaw, budgerigar, lories, and lorikeets.
Young birds may have navel illness. Signs may be insidious and nonspecific. The bird may fail to thrive or have ill thrift, but no signs are pathognomonic.
Diagnosis in the live bird relies on diagnosis of bacterial infection based on culture of the affected area, blood culture, or fecal culture. Gram stain of the affected system that shows heavy growth of gram-negative rods may be supportive of the diagnosis. Classically, granulomas are found in the intestinal tract and liver at necropsy.
As a normal component of GI flora in most humans, it is of minimal zoonotic risk.
Prompt, appropriate antibacterial treatment and aggressive supportive care are important in these cases, as the birds are often found severely compromised, septic on presentation, or both.
Affected birds should be isolated until the infection is resolved. Glove use and appropriate sanitation and disinfection are required.
Sodium hypochlorite is an effective disinfectant.
Mycobacteriosis
M. genavense
M. avium
M avium intracellulare complex
M. tuberculosis
Zoonotic potential depends on mycobacterial species identified.
All parrots susceptible; immunocompromised individuals are at most risk.
Many mycobacteria are common soil saprophytes or found in water sources.
Transmission is generally considered to occur by the fecal–oral route.
Classic clinical signs are that of a bird with ravenous appetite, which continues to lose weight, as mycobacterial disease most often affects the GI tract in birds. However, clinical signs are often insidious and may mimic many other diseases. Signs may occur in any system, including the respiratory system, eyes, skin, muscle, and bone. Common signs include dysfunction and enlargement or nodular swelling in the affected area.
Acid-fast staining of feces or affected organs; histopathology and acid-fast staining, PCR, and culture remain the gold standard but are expensive and slow.
Diagnosis may be made on the basis of associated complete blood cell count (CBC) (often, but no guarantee of elevated white blood cell [WBC] count >30,000) but must be confirmed by biopsy or cytological sampling and PCR or culture of the affected organ, usually at necropsy.
Affected organs at necropsy (classically the liver and spleen) may be found enlarged, pale, or both and have white-yellow nodules.
Although the zoonotic potential of parrot-associated mycobacteriosis appears very low, the public should not be exposed to infected parrots.
Treatment with multidrug therapy, similar to that of humans, has been attempted in some parrots.
Preventive measures include obtaining a full history on any birds donated to the collection and limiting contact with free-living birds.
Specific disinfectants available and labeled as mycobacteriocidal should be used in bird quarantine areas.
Diagnosis of mycobacteriosis in the live bird is challenging, and a single negative screening sample (usually obtained from the liver) does not guarantee that the animal is free of mycobacterial disease.
Pasteurellosis
P. multocida
Small gram-negative coccobacillus.
Nonmotile, penicillin-sensitive, facultative anaerobe.
Parrots appear exquisitely sensitive to a variety of bacterial pathogens, most importantly P. multocida, found in the mouths of predators. Parrots are unlikely to survive for more than 24 hours after predator attack because of overwhelming sepsis, likely to occur without antimicrobial support.
Parrots maintained outside are often mauled through the cage as they sleep. The next morning they may be miraculously found to be alive because of the effects of shock, only to perish from overwhelming sepsis a few days later if appropriate and aggressive therapy is not instituted. Drooping or missing appendage(s), blood in the enclosure, and multiple missing feathers are classic presenting signs. Absence of bite marks does not negate the possibility that a predatory attack has occurred. Avian stoic behavior, feathers, and skin are very good at obstructing the health care professional from recognizing serious damage and inflammation.
Because of the extremely unstable nature of many of the cases, extensive diagnostics are often forgone; a presumptive diagnosis and aggressive supportive care, including hydration, warmth, and appropriate antibiotic administration, which should initially be parenteral and include a gram-positive spectrum, are provided. Flouroquinolones alone may not provide adequate gram-positive antibacterial spectrum for these cases.
CBC may demonstrate left shift and leukopenia in severely affected cases, as well as anemia from blood loss.
Many cases with severe damage to the appendage may require amputation to resolve local infection.
Zoonotic potential is low (humans obtain the organism from bites or scratches from domestic pets) from the parrot, but gloves should be worn when handling any parrot wound or abrasions to minimize colonization by normal human bacterial flora.
Provide predator-proof enclosures, especially at night.
Prompt attention and aggressive antimicrobial and supportive care to these cases is essential for the best outcome. Amputation of the affected portion of the limb should be considered in refractory cases that have been stabilized. Immediate anaerobic and aerobic cultures are indicated.
As a microaerophilic bacterium, this organism is unlikely to survive for long in the environment, However, polymicrobial infections are common in cases of animal attack; therefore, wound cover and glove use, as well as enclosure disinfection with steam, are recommended.
Salmonellosis
Salmonella spp.
Gram-negative bacterium.
In captivity salmonella carriage and salmonellosis are common in parrots; however, prevalence in wild parrots appears low. Transmission is by the fecal–oral route. Food contamination is common.
Clinical signs include those related to sepsis (lethargy, depression) and those related to gastroenteritis (anorexia, weight loss etc.).
Birds may die acutely with minimal clinical signs.
Diagnosis may be challenging in the live bird, as culture is the gold standard but requires selective media; and Salmonella spp. are intermittently shed. Birds may remain carriers for prolonged periods without clinical signs, although this has not been specifically proven in parrots.
A bird with consistent clinical signs, and fecal Gram staining consistent with gram-negative bacterial infection should prompt cloacal bacterial culture collection immediately prior to institution of treatment.
Salmonellosis is a zoonotic pathogen.
Affected birds should be isolated until the infection resolves. Treatment should include hydration support and antibacterials if leukocytosis and signs of illness or sepsis are present. Caretakers should wear gloves when handling birds and masks when washing down enclosures. Appropriate sanitation and disinfection, including hand washing, are required.
Birds used for educational display and that may come in direct or indirect audience contact should be screened, more than once, for salmonellosis. Parrots should be kept separate and handled separately from raptors and reptiles.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Psittaciformes
Chapter 21
