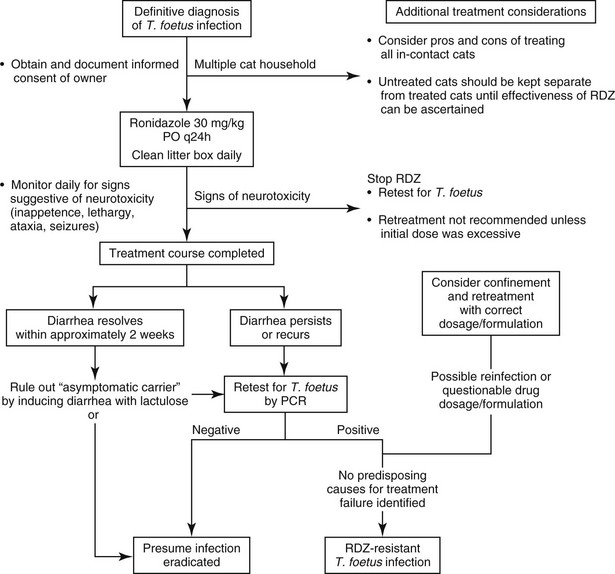
Chapter 129 Dietary trials, homeopathic remedies, corticosteroids, and most classes of antimicrobial drugs have been ineffective in ameliorating signs of diarrhea or eliminating T. foetus from infected cats (Foster et al, 2004; Gookin et al, 2010). Trichomonads generally are susceptible to the effects of 5-nitroimidazoles, because the organisms use anaerobic metabolic pathways that reduce these drugs to cytotoxic nitro anions capable of disrupting protozoal DNA. Prevalent resistance of feline T. foetus to metronidazole is presumed on the basis of common treatment failures with this drug, which has prompted investigation of related 5-nitroimidazoles such as tinidazole and ronidazole for treatment of the infection (Gookin et al, 1999, 2006; Kather et al, 2007). Tinidazole at high doses fails to consistently eradicate the infection from experimentally infected cats (Gookin et al, 2007) and has not been rewarding for treatment of naturally infected cats. Ronidazole (RDZ) is the only antimicrobial for which convincing efficacy for treatment of T. foetus infection has been demonstrated both in vitro and in vivo (Gookin et al, 2006). Pharmacokinetic studies of RDZ after oral administration to healthy cats demonstrate rapid drug absorption, high bioavailability, and a slow elimination half-life consistent with once-a-day dosing recommendations (LeVine et al, 2011). Experientially, RDZ has a narrow margin of safety between the minimum therapeutic dose and minimum toxic dose, leading to strict recommendations that cats be treated with 30 mg/kg administered orally once a day. It is recommended that cats be treated for a maximum of 14 days; however, the possibility that shorter durations of treatment may be equally effective has not been examined. The most common adverse effect of RDZ is dose-dependent neurotoxicity that ranges in severity from lethargy and inappetence to ataxia and seizures (Rosado et al, 2007). The most common preventable causes of toxicity are the administration of higher-than-recommended doses of RDZ and continued administration of RDZ despite subtler signs of toxicity. RDZ should be avoided in cats with systemic illness that could confound recognition of adverse drug effects and should not be given to pregnant or nursing queens or their unweaned kittens. RDZ is not registered for human or veterinary use in the United States. Therefore treatment with RDZ should be considered only in cases of confirmed T. foetus infection, in which informed consent has been obtained. Several pharmacies compound chemical grade RDZ for veterinary use. Because of its foul taste and undetermined stability, compounding into gelatin capsules rather than flavored liquids is recommended. Several drug formulations for treatment of birds can be obtained online without prescription from pigeon supply warehouses. Because of their undetermined quality, composition, and low active-drug concentration, these products are not recommended. Determining whether RDZ has eradicated or merely concealed T. foetus infection in any given cat remains an area of frustration (Figure 129-1). If diarrhea persists or recurs at 2 weeks or more after completion of RDZ, the author recommends that cats be retested for T. foetus by means of PCR performed on a fecal sample collected by the colon saline flush technique (www.JodyGookin.com). If this test result is negative in a cat with diarrhea, then persistent infection is considered unlikely. Repeat testing with confirmatory negative results would further support this conclusion. The greatest difficulty arises in confidently ruling out persistent infection in a cat that no longer has diarrhea after treatment with RDZ. In the author’s experience, periods of asymptomatic infection are not uncommon in T. foetus–infected cats and can be difficult to diagnose. In cases in which confirmation of T. foetus eradication is particularly relevant (e.g., reintroduction of a treated cat into a cattery), the author treats the cat with lactulose “to effect” to induce a soft diarrhea and then tests a colon saline flush sample for T. foetus by means of PCR. Negative test results in this instance would come as close as possible to confirming the absence of infection. 1. The cat was not effectively administered the recommended dose, duration, or formulation of RDZ. Treatment of cats with gel capsule–compounded pure RDZ at the recommended dosage typically prevents any second-guessing about drug quality and delivery. 2. Treatment failure could be attributed to reinfection during or after treatment with RDZ. A common misconception is that asymptomatic cats are not infected with T. foetus. When presumably uninfected cats are allowed contact with cats that later fail treatment with RDZ, reinfection (whether likely or unlikely) can never be ruled out. This possibility can be prevented by confining T. foetus–infected cats during treatment and until their treatment outcome can be assessed. 3. Treatment failure can be attributed to infection with a strain of T. foetus that is resistant to RDZ (Gookin et al, 2010). The prevalence of RDZ-resistant T. foetus infection in cats is unknown but is suspected to be significant. Although resistance can be documented in the laboratory, it can be assumed if treatment failure is observed in a cat that receives the appropriate dosage of RDZ and has not been exposed to other cats during or after treatment. In the author’s experience, higher doses, more frequent administration, or longer durations of treatment with RDZ have been ineffective in eradicating T. foetus from such cats and directly increase the risk of neurotoxicity. The high level of existing clinical resistance of feline T. foetus to metronidazole, low efficacy of tinidazole, and documentation of resistance to ronidazole in some cats are consistent with a high level of cross-resistance to 5-nitroimidazole drugs among feline T. foetus. The current lack of alternative drugs with clinical efficacy against feline T. foetus infection suggests that active investigation of other treatment approaches is warranted. When left untreated, 23 out of 26 (88%) cats with T. foetus infection were reported to undergo spontaneous resolution of diarrhea within 2 years (median 9 months; range 5 months to 2 years) (Foster et al, 2004); however, most remained infected based on PCR results when retested as long as 2 to 5 years after initial diagnosis. The role of these “asymptomatic carriers” in disease transmission is unclear; however, such cats can undergo a full relapse of clinical trichomonosis as long as 6 or more years after onset of clinical remission. Accordingly, any cat harboring T. foetus should be considered a liability for transmission of infection, and detection of such cats for the sake of preventing disease transmission appears warranted. At present no evidence or studies have been conducted to examine for any long-term adverse health effects of asymptomatic T. foetus infection in the cat.
Protozoal Gastrointestinal Disease
Tritrichomonas foetus
Ronidazole
Establishing Treatment Efficacy
Causes and Prevention of Treatment Failure
Ramifications of Not Treating
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Protozoal Gastrointestinal Disease
Only gold members can continue reading. Log In or Register to continue