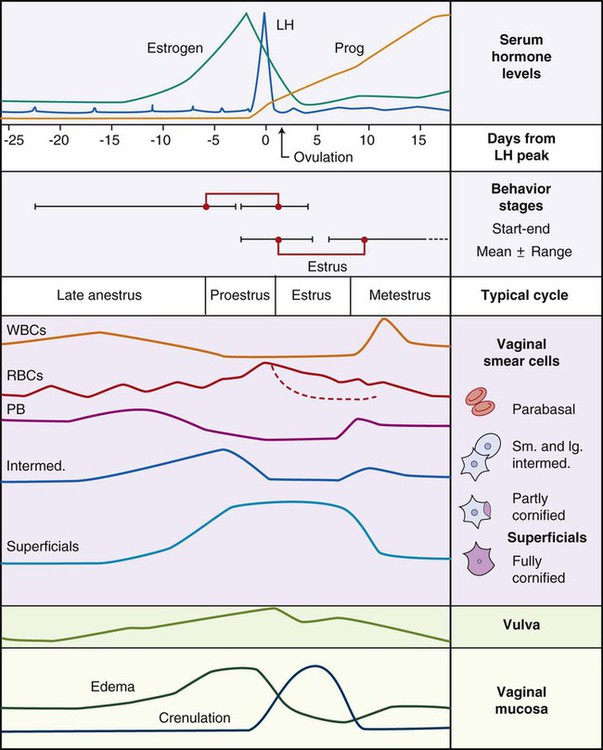
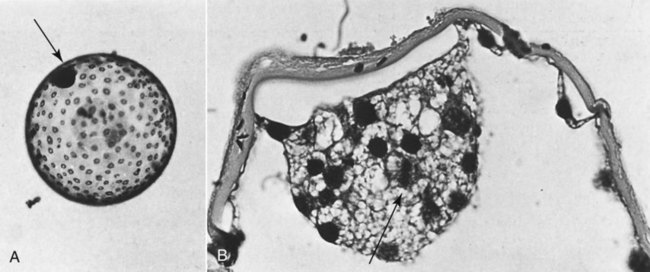
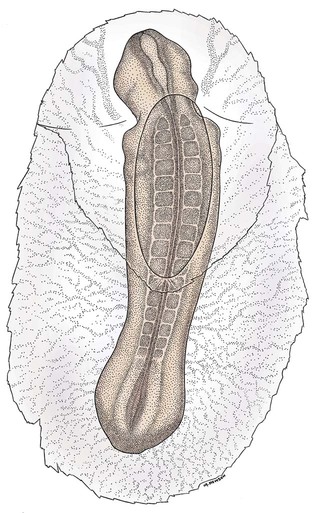
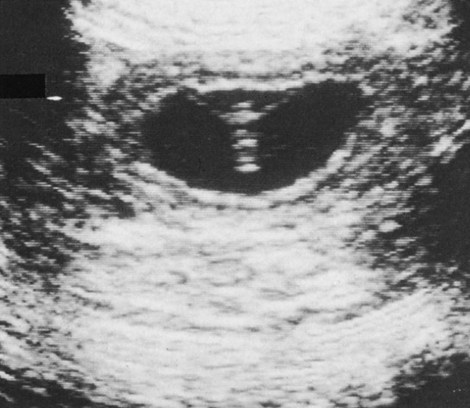
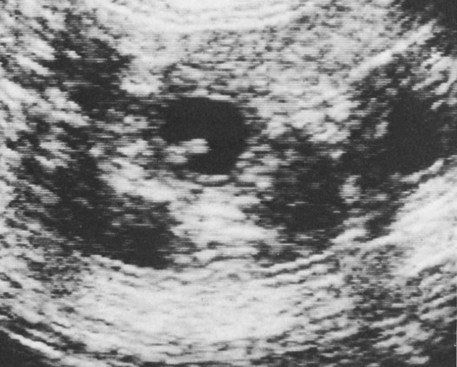
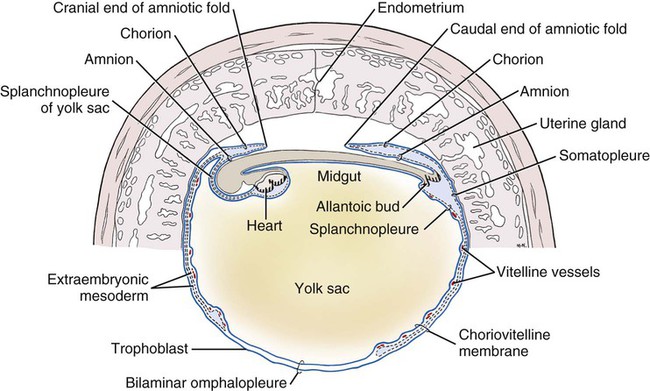
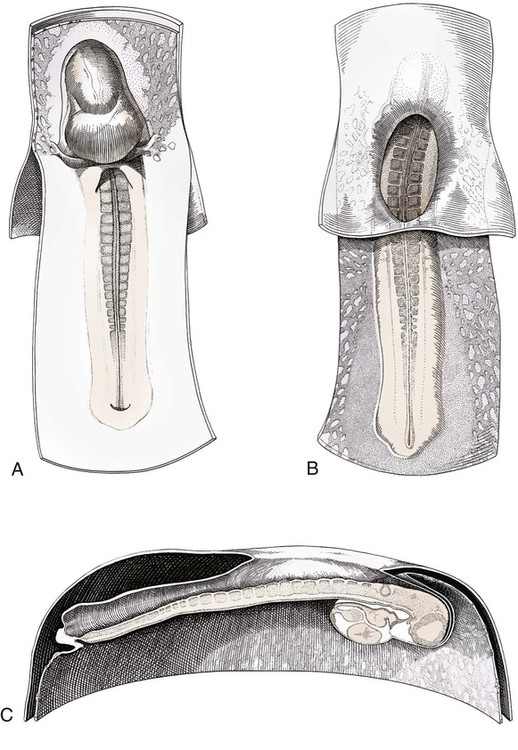
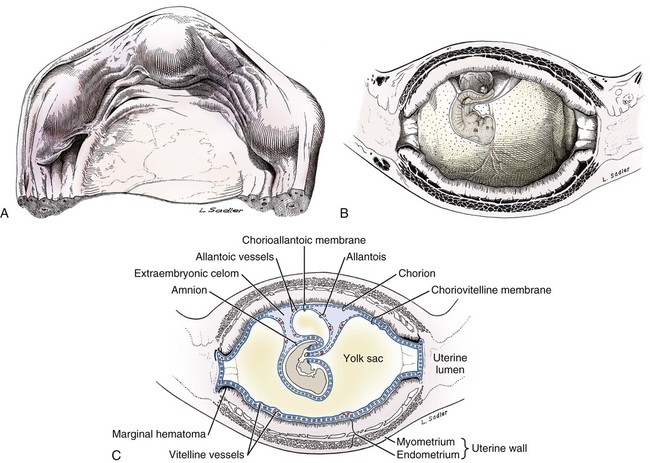
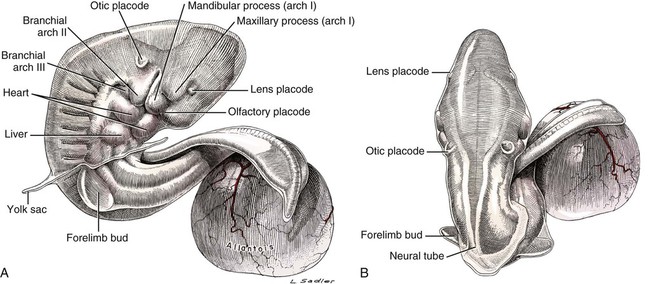
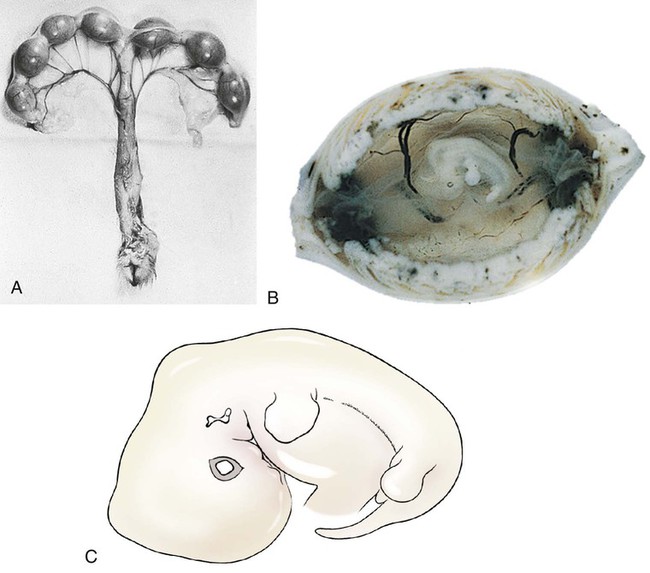
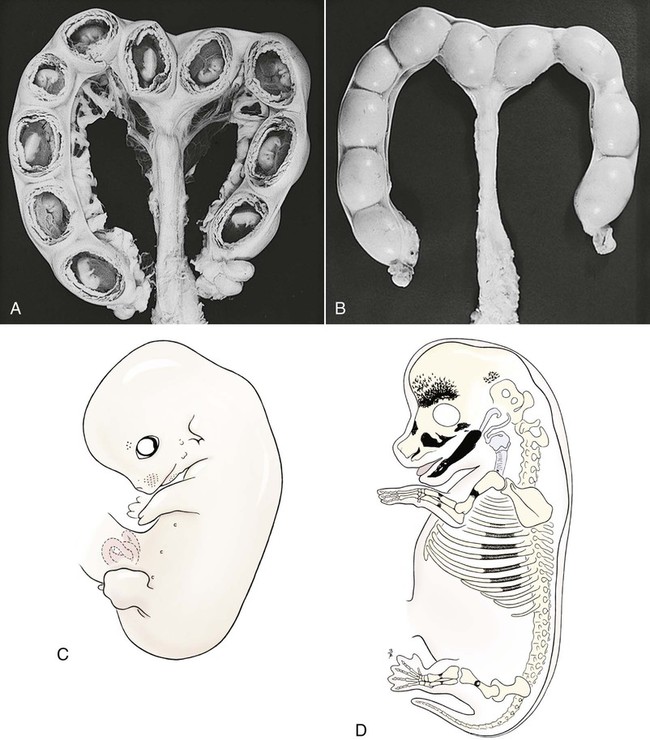
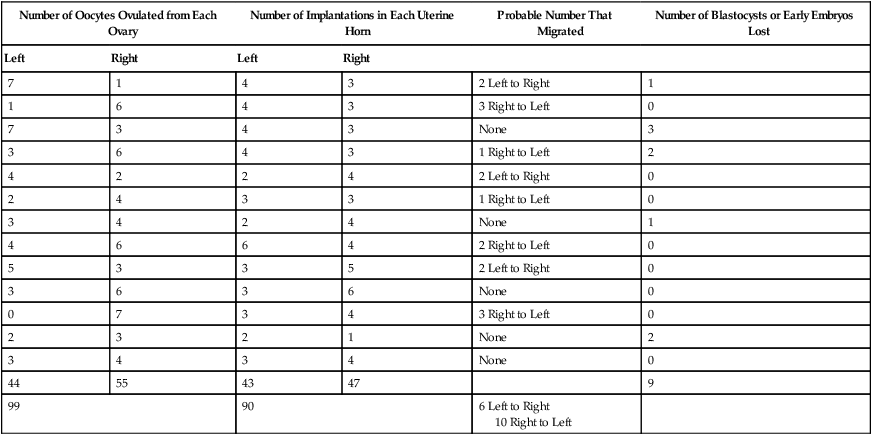
Ovulation in the dog occurs spontaneously early in estrus, as was shown by Bischoff in 1844. The germ cell of the dog at this time (unlike most other mammals) is a primary oocyte, because the first and second polar bodies are not formed until after fertilization. A recently ovulated oocyte with its covering of corona radiata cells can be seen with the unaided eye and is approximately 230 µm in diameter. Photographs of preimplantation ova have been published by Holst and Phemister (1971). The induction of estrus and fertile ovulations was studied by Concannon et al. (1997). A study of ovulation, fertilization, and early development in the dog was reported by Renton et al. (1991). Passage of spermatozoa through the uterus and tubouterine junction and up the uterine tube can be rapid in the dog. Bischoff (1844) noted that it takes 6, 18, or 20 hours for sperm to enter the uterine tube. Doak et al. (1967) found motile spermatozoa in high concentration in all parts of the dog’s uterus for 6 days after copulation, and some were present for as long as 11 days. Holst and Phemister (1974) confirmed at least a 7-day life span of dog spermatozoa. It is thought that the fertile life of a sperm is approximately half of its motile life, and this long fertile period may be responsible for the high conception rate in dogs. The nuclear chromosomes of the sperm become the male pronucleus, which fuses with the female pronucleus of the zygote to determine the genetic constitution of the zygote. The dog has a great number of small chromosomes, which makes it difficult to demonstrate all of them in a single preparation even after cell culture. Moore and Lambert (1963) illustrated and described the karyotype of the Beagle from an analysis of several metaphase plates taken from cultured kidney cells. They confirmed the findings of Minouchi (1928) and Ahmed (1941) that the diploid number in somatic cells of the dog was 78 in both sexes. The chromosomes appear to be aligned into 38 homologous pairs of autosomes and two sex chromosomes. All of the autosomes have acrocentric or terminal centromeres. The X chromosome is one of the largest, whereas the Y chromosome is equal to the smallest. The X chromosomes have metacentric centromeres. For a discussion of genetics in the dog, see Asdell (1966). For a wealth of information on the genetic basis of breed characteristics in purebred dogs see the monograph of Stockard (1941) who noted striking reproductive peculiarities during the course of his cross-breeding program. Certain of the giant breeds were poor producers, either failing to whelp or producing only one to three pups. In contrast, closely related dogs produced from 10 to 17 puppies at a whelp, and one of his bitches produced 50 puppies within 2 years. Songsasen, Spindler, and Wildt (2007) studied in vitro cultivation of dog oocytes and pointed out that although intraovarian oocytes can be recovered, matured, and fertilized in vitro (Rodriques & Rodriques 2006), embryo transfer has never been consistently successful in the dog. Such methods will allow the rescue of genetic material from valuable genotypes of domestic and wild canids. Songsasen and Wildt (2005) showed that the size of the donor follicle was most important in determining meiotic competency during follicular development. Hewitt and England (1999) studied the maturation of oocytes in vitro. The extensive endocrinologic investigations of Concannon and his colleagues, summarized by Concannon (1991), have shown that the average onset of estrus in the dog is 1 day after the luteinizing hormone (LH) surge, which can be identified by a rise in serum progesterone in the blood (Fig. 2-1). They reported increases of 20 to 40 times in the level of LH (8 to 50 ng/mg with an average of 20 ng/mg) during the 1- to 2-day preovulatory surge. The average time of ovulation was day 2 of estrus. However, there were ovulations before behavioral estrus, early in estrus, and in late estrus. The peak of fertility for natural matings ranged from 1 day before the preovulatory LH surge until 5 or 6 days after the LH surge. When Evans (1956) was breeding Beagles for the collection of the embryos and fetuses used in this chapter, the method of using the LH surge as the most fixed point in the estrous cycle had not been described. The method used to determine gestation times, as cited in the accompanying tables and figures, was to examine the bitches daily for signs of estrus and to test how they reacted to the stud. When the bitch would stand for the stud without snapping or sitting down, she was isolated and bred 24 hours later. Using this system of a single mating for each estrus, in a colony of 19 purebred Beagles, all single matings in the first year were successful. From these matings, 24 hours after first acceptance, embryos or fetuses were surgically removed singly or in groups at various intervals for study. A single stud was used for 6 years, and the average litter size from this inbred colony of 20 Beagles was 6.7 pups. Bitches came into heat at all times of the year, although there was a peak in the spring and summer period. Some of the largest litters were from the youngest bitches. According to Concannon (1991) maximum receptivity of the bitch is seen at the peak of the LH surge, and ovulation usually occurs 2 or 3 days later. Using his system of timing gestation, day “0” is the day of the LH surge, and birth occurs 65 days later, which would be 62 to 63 days after ovulation. Holst and Phemister (1974) observed that the diestrous period (now called metestrus) was closely correlated with the length of gestation, and they calculated gestation time by backdating to the onset of this period. Metestrus is the last period of the luteal phase, when the bitch no longer stands for the stud. The beginning of this period is characterized by a marked shift in the epithelial cell type seen in the vaginal smear. On the first day of metestrus there is a decrease in the number of cornified cells and an increase (often more than 50 per cent) in noncornified cells from the deeper layers of the vaginal epithelium. When the onset of metestrus was used as a fixed point to backdate the early stages of embryonic development, Holst and Phemister (1971) made the following correlations: Metestrus minus 1 day = the two-cell cleavage stage M minus 3 days = fertilization M minus 4 days = formation of secondary oocyte M minus 5 days = beginning of meiosis They suggested that if the day of fertilization is counted as “day 1,” then the Beagle has a gestation period of 60 days, and whelping takes place on day 57 of metestrus. Holst and Phemister (1974) found that the highest conception rate could be attained by two matings: one at the time of first acceptance and the other on the third day or later. The use of doppler and A-mode or B-mode ultrasound for pregnancy diagnosis in the dog showed the usefulness of this noninvasive technique, which is approximately 99% accurate (Cartee & Rowles, 1984; Shille and Gontarek, 1985; Toal et al., 1986; Taverne & van Oord, 1989). With B-mode ultrasound many features of the conceptus, embryo, and fetus can be recognized in greater detail (England and Allen, 1990; England et al., 1990; Yeager et al., 1992). Yeager and Concannon (1990), using ultrasonography for detection of early pregnancy, cited the embryonic heartbeat as the earliest detectable sign of a viable pregnancy. They first detected the heartbeat at 23 to 25 days after the LH surge, at which time the embryonic mass was 1 to 4 mm in length. They found that the correlation of first breeding and ultrasonic detection of pregnancy was more variable than the correlation between LH surge and the ultrasonic detection of pregnancy. Yeager et al. (1992) summarized the ultrasonic appearance of the uterus, placenta, fetus, and fetal membranes throughout gestation in the Beagle. For timing gestation they used the day of the LH surge as “day 0,” as described earlier. Their investigations showed the earliest detection of the chorionic cavity at day 20; embryo and heartbeat at day 23 to 25; yolk sac membrane at days 25 to 28 (see Figs. 2-5 and 2-6); allantoic membrane at days 27 to 31; and skeleton at days 33 to 39. Of the fetal structures, head diameter was the best indicator for estimation of gestational age. The timing of breeding using the behavior of the bitch, vaginal cytologic examination, or both as guides, although less precise than timing from an LH assay, can also result in good correlations with gestation age, judging from the similarity of development of embryos and fetuses at any given date and their stages of skeletal development (Evans, 1958–1974). By surgically removing embryos or fetuses at various intervals during gestation, it was possible to measure individuals or entire litters from the same dog. Knowing the interval between removals allowed for the calculation of growth rates. The data and specimens collected for this study are available in the Cornell Embryo Collection, Department of Biomedical Sciences at the College of Veterinary Medicine. As an example of incremental growth changes that can be seen, a bitch with 10 conceptuses was operated on at 35 days postinsemination, and five fetuses were removed from the right horn that averaged 41 mm in crown-rump (C-R) length. On day 40, five fetuses were removed from the left horn that averaged 70 mm in C-R length. This average increase of 29 mm in 5 days indicated a growth rate of approximately 6 mm a day (Evans, 1959). Thus there can be a considerable discrepancy in the size or morphologic characteristics of an embryo or fetus if the estimated gestation time is off by a day or two (Table 2-1). TABLE 2-1 Transuterine Migration of Blastocysts in 13 Beagles The successive stages of cleavage, gastrulation, implantation, and early somite formation are completed before day 20 of gestation. The length of time required for the entire development of a fertilized oocyte into a newborn puppy is approximately 60 days, and it is no wonder that marked changes in development can be seen at daily intervals. Phemister (1974) divided prenatal development in the dog into three periods: (1) the period of the ovum, following fertilization, which is characterized by a blastocyst (Fig. 2-2), which lies free in the uterine tube and migrates to the uterus (days 2 to 17); (2) the period of the embryo, which begins with implantation of the blastocyst (Fig. 2-3) and ends with the completion of major organogenesis (days 19 to 35); (3) the period of the fetus, the time during which the characteristic features of the dog appear and most of the growth occurs (day 35 to birth). Fertilization takes place in the cranial part of the uterine tube (oviduct), and the fertilized oocyte (zygote) begins to divide within a few hours. Tubal transport of oocytes/embryos takes longer in the dog (7 to 10 days) than in most other mammals (3 to 4 days), although some embryos reach the region of the tubouterine junction in approximately 5 days. Holst and Phemister (1971) found that developing embryos may enter the uterus as early as the 16-cell stage but more commonly as morulae or even early blastocysts. This agrees with the observations of Anderson (1927) and Van der Stricht (1923). Estrogen inhibits the transport of developing embryos into the uterus, whereas progesterone enhances it. Within the uterus the morula develops into the rather spherical blastocyst, with an inner cell mass and a thin surface trophoblast surrounded by a zona pellucida (see Fig. 2-2). Unattached blastocysts range in size from 250 µm to 3 mm as they pass along the uterine lumen prior to implantation on about day 17 after breeding. The free-floating blastocyst stage lasts approximately 7 days (Gier, 1950; Holst & Phemister, 1971; Tietz & Selinger, 1967). There is usually an even spacing of blastocysts in each uterine horn, but there is often a marked difference in the number of oocytes ovulated from each ovary, as ascertained by the number of corpora lutea seen. Thus oocytes ovulated by one ovary and fertilized in the cranial end of the uterine tube on that side may migrate through the body of the uterus and traverse the opposite uterine horn to implant. The early studies of Bischoff (1844) on the dog called attention to the phenomenon of transuterine migration, as did later studies in the cat by Hill and Tribe (1924) and other carnivores by Boyd et al. (1944). Evans (1956) examined the ovaries and uterine horns of 13 bred Beagles for evidence of transuterine migration and found that in 8 dogs a total of 16 morulae or blastocysts had crossed from one uterine horn to the other. There was no way of telling how many others had changed sides without affecting the final balance, because no marker was used to distinguish right from left ovulations (see Table 2-1). See Box 2-1 for a summary of the morphologic events during the first trimester of development. The implanting blastocyst on day 18 of gestation lies in a pear-shaped cavity of the uterus that is hardly discernible on external examination (see Fig. 2-3). The lumen between successive implantations is very narrow, and therefore implantation sites can be readily identified on dissection. Fluid that collects within the blastocyst causes it to expand and fill the small uterine cavity. The attachment process, referred to as central implantation, is a superficial apposition of trophoblast to the antimesometrial surface of the uterine endometrium. The trophoblast cells are responsible for initiating formation of the placenta, and in the dog, they elaborate the enzymes that erode the maternal tissues to form the definitive endotheliochorial deciduate zonary placenta. Barrau et al. (1975) studied early implantation in the dog and found the invasive form of trophoblast to be syncytial, as did Schoenfeld (1903), rather than cellular, as reported by Amoroso (1952). Three types of trophoblast are ultimately recognizable: (1) syncytial trophoblast around maternal blood vessels; (2) cytotrophoblast in the necrotic zone; and (3) phagocytic cytotrophoblast around the hematomata. In addition to the variety of absorptive cells present in the dog’s placenta, there are occasional decidual cells associated with the trophoblast of the labyrinth (Anderson, 1969). In some species the trophoblast is primarily nutritive and hormone-producing, whereas in others it is very invasive. In the dog it is invasive and syncytial. The embryo develops in a cephalocaudal sequence, beginning with head folds, then neural tube closure, followed by somite formation, appearance of branchial clefts, lens placode, otic placode, cardiac bulge, and the growth of limb buds. For specifics on domestic animal embryologic development, including the dog, see Noden and de Lahunta (1985), McGeady, TA et al., Veterinary Embryology (2009) and Hyttel, P et al., Essentials of Domestic Animal Embryology (2010). Several days after implantation, the developing embryo is enveloped by amniotic folds that grow across its dorsal surface (Fig. 2-4). At the 21-day stage (16 somites, 4.5 mm), the embryo is crescent-shaped, and the yolk sac, which is continuous with the midgut, rapidly fills the chorionic vesicle (Figs. 2-5 to 2-7). Where the trophoblastic ectoderm and yolk sac endoderm are in apposition on the mesometrial wall of the uterus, it is called the bilaminar omphalopleure. Closer to the embryo, where the extraembryonic mesoderm and its vitelline vessels extend between the trophoblast and the yolk sac endoderm, a trilaminar omphalopleure or choriovitelline membrane is formed that serves as a transitory placenta. The amnion of the dog develops by outfolding after the embryo is well organized. This is unlike the process in the human, in whom the amnion develops by cavitation of the inner cell mass in the presomite embryo prior to the establishment of germ layers. By day 21 in the dog, the amniotic folds are well developed over the dorsum of the embryo, and the vitelline vessels are extensive (Fig. 2-8). The amnion fills with fluid as the embryo grows and, in later fetal stages, becomes a sizable sac. By day 23, when the uterine swellings are almost spherical (Fig. 2-9A), the embryo has approximately 32 somites and is approximately 5 mm long. The yolk sac is very extensive, closely applied to the chorion, and well vascularized (see Fig. 2-9B). The vascularity, as well as the size of the yolk sac, increases throughout gestation, although it is superseded functionally by the allantois on about day 25 of gestation. The allantois, a diverticulum of the hindgut, is first visible as a bud on day 21, and by day 23 it is a spherical sac located ventral to the caudal end of the embryo and projecting into the exocoelom (see Fig. 2-9C). As it progressively expands, the allantois contacts the chorion (somatopleure) over most of the exocelomic surface, and allantoic blood vessels can be seen on its surface. By day 25 the allantois has intercalated itself between the chorion and the body of the yolk sac, thus completing the formation of the definitive chorioallantoic membrane. The enveloped yolk sac is separated from the chorion except at the poles, where it remains attached. The choriovitelline placenta that exists from days 21 to 23 has simple villi that project into the openings of the endometrial glands. The villi are avascular and thus likely serve a histiotrophic function, as suggested by Wimsatt (1974), who studied the 23-somite black bear. As the placenta grows, the uterine swelling changes from pear-shaped (20 days) (see Fig. 2-3) to spherical (25 days) to ovoid (30 days) (see Fig. 2-9). During this time the thickening of the uterine glands and the rapid expansion of the allantoic sac result in a change from a primary choriovitelline (yolk sac) placentation to the definitive chorioallantoic placentation. Embryos appear most vulnerable to physiologic stress during this period of rapid growth and placental change. Pressures within the uterus are greatest when the allantoic sac has expanded and the uterine swellings are spherical or beginning to elongate on day 25 of gestation. Death of the embryo prior to day 25 usually results in complete resorption with little or no visual evidence at term of the placental site. Death of early embryos is manifested by a reduced litter size but can be confirmed only by examination of the interior of the uterus. Most carnivores have marginal or central blood sinuses or hematomata associated with the placenta (Creed, 1963). The dog has a marginal “green band,” which can first be recognized in early limb-bud stages as a slightly thickened region between the choriovitelline membrane and the endometrium at the constricted ends of each uterine loculus (see Fig. 2-9C). This incipient marginal hematoma thus appears simultaneously with the expansion of the allantoic sac but prior to allantoic contact in the marginal region. The green band at each end of the zonular placenta increases in size throughout gestation (see Figs. 2-9C and 2-11B). Occasionally, there are isolated hematomatous patches in the central area of a normal dog placenta. These structures have been called hemophagous organs by Creed and Biggers (1963). Hematomata form by necrosis of both the maternal and the fetal tissues. Maternal vessels hemorrhage into the necrotic area and form pools of blood with a greenish color. These hematomata are surrounded by trophoblast rather than being between cytotrophoblast and maternal endometrium (Barrau et al., 1975). Duke (1946) described developing monozygotic twins (10 mm C-R) in a dog that had eight other normal conceptuses. The embryos were in a limb-bud stage. Although monozygotic twins occur commonly in some species, they are rare in carnivores, and this was the first description of such embryos in the dog. (Evans examined more than 400 pregnant uterine horns and never found more than one embryo or fetus in a single loculus.) The two embryos described by Duke shared a placenta, were enclosed by a single chorion, and had a common yolk sac. Each embryo had its own amnion, indicating that the twinning process had occurred before amniogenesis. See Table 2-2 for a summary of the morphologic events during the second trimester of development. TABLE 2-2 Summary of Morphologic Events During the Second Trimester of Development Streeter (1951) divided the human embryonic period into 23 “horizons” based on both external and internal features. Following the 23rd horizon was the fetal period, characterized in the human by the appearance of bone marrow in the humerus. This occurred in the 28- to 30-mm embryo at approximately 7 weeks of age. During the fetal period, linear dimensions are used for the assessment of age, the commonest being C-R, or sitting height, and crown-heel, or total length, for man. In domestic animal embryos and fetuses the most widely used measurement is the straight line C-R length, which allows for continuous measurement during most of the prenatal period (Table 2-3). Evans and Sack (1973) plotted prenatal growth in several domestic animals, including the dog, and considered the fetal stage of the dog to begin on day 35, when the digits on both limbs were fully formed and the external features were clearly those of a dog (Fig. 2-13). TABLE 2-3 Crown-Rump Length Estimates for Each Day of Gestation in the Beagle
Prenatal Development
Early Development
Length of Gestation
Number of Oocytes Ovulated from Each Ovary
Number of Implantations in Each Uterine Horn
Probable Number That Migrated
Number of Blastocysts or Early Embryos Lost
Left
Right
Left
Right
7
1
4
3
2 Left to Right
1
1
6
4
3
3 Right to Left
0
7
3
4
3
None
3
3
6
4
3
1 Right to Left
2
4
2
2
4
2 Left to Right
0
2
4
3
3
1 Right to Left
0
3
4
2
4
None
1
4
6
6
4
2 Right to Left
0
5
3
3
5
2 Left to Right
0
3
6
3
6
None
0
0
7
3
4
3 Right to Left
0
2
3
2
1
None
2
3
4
3
4
None
0
44
55
43
47
9
99
90
6 Left to Right
10 Right to Left
Prenatal Periods
Oocyte—Embryo
Embryo
Bitch
Embryo
17-18 days
Uterus same size as in a pseudopregnant dog.
Blastocysts evenly spaced from one another, trophoblastic attachment, zona pellucida shed, primitive streak visible, caudal to neural plate.
20 days
Implantation chamber of the uterus is a pear-shaped cavity.
8 somites; 4 mm long; neural tube closing.
21 days
Uterus slightly enlarged at placental sites.
16 somites; 5 mm long and crescent-shaped; longitudinal axis of embryo transverse to uterine horn; head flexed invaginating yolk sac; cardiac bulge prominent; allantoic bud developing; amniotic folds closing; yolk sac fills uterine cavity and is widely confluent with the midgut; branchial arches I and II present.
23 days
Uterine swellings distinct; vascular and glandular layers of the uterus hypertrophied (see Fig. 2-9A to C).
32 somites; 10 mm long; twisted with cranial end invaginated into yolk sac; forelimb bud prominent; otic placode and lens placode present; mandibular and maxillary processes distinct; branchial arches I, II, and III present; liver bulge marked; allantoic sac spherical and beneath tail (Fig. 2-10).
25 days
Corpora lutea completely fill the ovary; uterine swelling almost spherical, 30 × 35 mm; width of placenta approximately 29 mm (Fig. 2-11A and B).
14 mm; cephalic flexure prominent; otic pit and eye well formed; mammary ridge present (see Fig. 2-11C); limb buds at plate stage; vertebral elements chondrify; dental lamina forms.
28 days
17 mm; first ossification seen in mandible, maxilla, frontal bone, and clavicle.
30 days
Uterine swellings 33 × 50 mm; width of placenta is greater than length of embryo (Fig. 2-12A).
19 mm; eyelids and external ear forming; sensory hairs on snout, chin, and eyebrow; intestine herniated into umbilical stalk; five pairs of nipples; digits on forelimbs distinct; genital tubercle prominent (see Fig. 2-12B).
33 days
27 mm; ossification of nasal, incisive, palatine, zygomatic, and parietal bones; midshaft of ribs 4 through 10; midshaft of humerus, radius, and ulna; femur, tibia, and fibula; canine teeth in early cap stage; palatal shelves fuse; digits on hindpaws distinct (see Fig. 2-12C).
Age Determination
Day
Total Length
18
1-2 mm
19
2-3 mm
20
4 mm
21
4.5 mm
22
4.5-5 mm
23
5-6 mm
24
10 mm
25
14 mm
26
15 mm
27
16 mm
28
17 mm
29
18 mm
30
19 mm
31
20 mm
Day
Crown-Rump Length
32
25 mm
33
30 mm
34
32 mm
35
35 mm
36
41 mm
37
47 mm
38
53 mm
39
59 mm
40
65 mm
41
67 mm
42
70 mm
43
75 mm
44
78 mm
45
86 mm
46
90 mm
47
95 mm
48
97 mm
49
100 mm
50
107 mm
51
120 mm
52
130 mm
53
138 mm
54
142 mm
55
144 mm
56
145 mm
57
150 mm
58
155 mm
59
157 mm
60
158 mm
63
165 mm ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Prenatal Development
Only gold members can continue reading. Log In or Register to continue