Chapter 2 Practical General Field Ophthalmology
Field Ophthalmology Basics
General Comments
Examination of the equine eye is simplified by the fact that the adnexa, globe surface, and intraocular contents are easy to inspect and often easy to image. Thorough examination can be done in the field if the practitioner arrives at the call with functional portable diagnostic tools and all the common drugs and supplies needed for routine restraint and handling. Examination will be proficient if the practitioner takes the time to create an environment conducive to proper examination, chooses effective patient position and restraint, images observable abnormalities with a digital camera, and follows the methodical examination techniques outlined in Chapter 1.
Although some ophthalmic problems are chronic, many are presented as ophthalmic emergencies. Horse owners and clinic reception staff must be trained to regard swollen, painful, traumatized, or acutely discolored eyes as true emergencies and schedule visits for all these cases on a same-day basis. Clients who call in with a horse that is suffering an ophthalmic emergency should be instructed to keep the animal in a clean, dark stall until it is examined. A workspace that can be darkened for examination and well lit for treatment should be available. The client should be instructed to gather the materials needed for a bale head support (4 to 6 bales of hay or shavings and a clean cover for the top bale) prior to the arrival of the clinician (Fig. 2-1). Clients should be cautioned against administration of medication in an eye that has not been examined.
Examination, imaging, and diagnostic testing must lead to a diagnosis. Ocular problems like trauma or overt neoplasia are obvious, but diagnosis of other problems can be challenging. The conditions most often misdiagnosed in the field are listed in Box 2-1; these conditions merit special study by all equine practitioners. Certain conditions, particularly diffuse nonulcerative loss of corneal transparency, glaucoma, and subclinical uveitis are not completely understood, so information on differential diagnosis is sparse. Other conditions such as stromal abscesses or fungal keratitis have diagnostic elements that may be overlooked or misinterpreted. Some problems like deep corneal infections and equine recurrent uveitis may present at a stage where multiple regions of ocular anatomy are altered, causing uncertainty as to which elements are primary issues and which elements are secondary. Improvement in diagnostic acumen is dependent on experience, constant study of new information, selection of appropriate diagnostic tests, and close surveillance of case progress and therapeutic effectiveness.
Equipping the Ambulatory Vehicle
The ambulatory practitioner must be prepared to diagnose and treat a wide variety of ophthalmic problems. Box 2-2 lists the equipment and supplies used in the diagnosis and acute treatment of common field conditions. Many common drugs that are routinely dispensed are easily obtained from veterinary distributors. However, serious or chronic ophthalmic conditions often require medication with drugs that are not carried by veterinary distributors. Box 2-3 lists drugs that usually need to be obtained through retail pharmacies or ordered through compounding pharmacies.
Box 2-3 | Ophthalmic Medications Available from Retail, Compounding, or University Pharmacies
Creating an “Exam Room” in the Field
Working on Painful or Fractious Animals
Flighty or very painful animals will require sedation for examination. Sedation will also be required on horses undergoing invasive or painful diagnostic or surgical procedures. Two drugs are commonly used for chemical restraint: xylazine (0.5 to 1 mg/kg) and detomidine hydrochloride (Dormosedan, 0.02 to 0.04 mg/kg). Detomidine produces more profound sedation than xylazine. Both take effect very quickly after intravenous (IV) administration. The exact dosage of drug and duration of sedation depends on the disposition of the animal, the existing level of pain, and the length of time and manipulation involved in any anticipated procedures. Variations exist among horse breeds in sedation effects: Draft breeds like Belgians and Percherons typically exhibit profound sedation at the lower end of the dosage spectrum, while high strung breeds like Arabians and Thoroughbreds may require higher doses. Please see Chapter 1 for more information on use of sedation for ophthalmic examinations.
Local and Regional Anesthesia
All practitioners should be skilled at performing auriculopalpebral and supraorbital nerve blocks as described in Chapter 1. Please see Chapter 1 for more information on performance of these nerve blocks.
Corneal or conjunctival topical anesthesia is necessary for many examinations and diagnostic tests. Short-term tissue desensitization is achieved by applying proparacaine onto the target surface. A dose of 0.5 mL per eye should be drawn up out of the dropper bottle into a small syringe using a 25-gauge needle. The needle should then be broken off and discarded and the anesthetic sprayed or dripped onto the corneal surface through the remaining hub. Topical anesthesia of focal areas on the conjunctiva or nictitans can be enhanced by briefly holding a sterile Dacron or cotton-tipped swab soaked in topical anesthetic against the target area. Duration of corneal desensitization may be brief in very painful conditions, so the examiner should be prepared to reapply anesthetic as needed.1
Performing Examinations in Field Settings
Detailed specifics of the ocular examination are described in Chapter 1. Several tips that may be helpful in field situations:
Life Stage Issues
Practitioners are responsible for the health of their patients throughout their lifespans. Surveillance for ophthalmic problems should begin shortly after birth. Ideally, ophthalmic exams should be performed regularly throughout the horse’s life. Equine practitioners should be familiar with the ocular problems commonly encountered during the various life stages listed in Box 2-4.
Box 2-4 | Equine Life Stage Ophthalmic Issues
Pediatric
Patient cooperation is optimized if one person holds the mare and another person holds the foal near the mare’s head. If two assistants are not available, most mares can be turned loose and will usually behave well as long as they can see the foal directly in front of them. Excessive foal restraint is inappropriate—eye examinations are best performed with the foal simply cradled by an assistant (Fig. 2-3). Rambunctious foals can be restrained with a firm arm around the chest and a tail hold. Sometimes it is helpful to position the foal in a corner or against the stall wall. The foal’s ventral mandible can be cradled with one hand while the other is used to hold the light source.
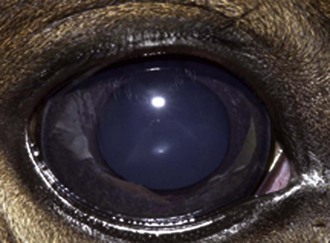
The neonatal pupil is somewhat round in contour. The globe has a mild ventromedial orientation at birth that gives neonates a slight downward gaze. The pupil acquires a horizontal elliptical shape and becomes parallel to the lower lid over the first month of life. Pupillary light response may be sluggish in the first few days of life but should be brisk after a few days. The color of the neonatal iris may be a little grayer than the rich chocolate color commonly seen in adults (Fig. 2-4). Lens suture lines may be very prominent and should not be mistaken for cataracts. Tapetal color is variable and is correlated with coat color. The optic disc is round to oval in shape and whitish pink to salmon in color. Light gray streaks, representing bundles of axons traveling to the optic nerve, may be seen in the peripapillary area.
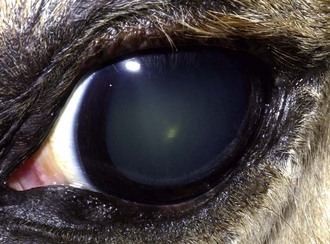
Mature horses frequently develop nuclear sclerosis as they age. This can give the lens a faint blue appearance that may be opaque on transillumination (Fig. 2-5). Geriatric horses often present with degenerative or age-related conditions of the eyes: cataracts, vitreal syneresis, asteroid hyalosis, senile retinopathy, supraorbital fat atrophy, or proliferative optic neuropathy. Horses older than 15 years are at increased risk for many kinds of neoplasia. Aged gray horses are at increased risk for melanoma and will occasionally present with ophthalmic forms of this disease.
Geriatric horses may have ocular manifestations secondary to chronic conditions such as dental disease, sinus infections, and Cushing’s syndrome. Diagnosis and management of equine geriatric ophthalmic problems have been reviewed,2 and more information on ocular manifestations of systemic disease can be found in Chapter 13.
Ophthalmic Tests in the Field
Field Tips for Vision Testing
Handlers and observers should be instructed to stay away from the maze line and avoid giving the horse any verbal cues to orientation. The horse being tested should be turned loose in the aisle. One person should stand at the end of the maze path and coax the horse to navigate the obstacles by shaking a bucket filled with grain (Fig. 2-6). Fractious horses may have to be restrained on a long lead, but the handler must avoid giving any positional cues to the horse.
Tips for Tonometry
Tonometry is discussed in Chapter 1. The two instruments most commonly used in the field are the Tono-Pen Vet applanation tonometer (Reichert Inc., Depew, NY <http://tonopen.com/>) and the TonoVet rebound tonometer (Icare Finland OY, Helsinki, Finland <http://www.icaretonometer.com/index.php?page=tonovet-for-animals>).
Field Tips for Ophthalmic Dye Tests
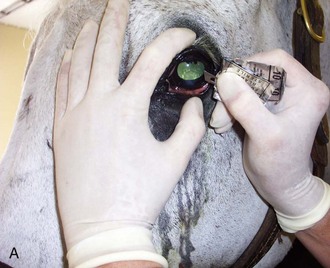
Ophthalmic dye testing procedures are discussed in Chapter 1. All eyes undergoing examination for a problem should undergo fluorescein dye staining. If eyewash is not available to mix with a test strip, sterile saline can be mixed with the torn-off paper dye strip in a syringe and sprayed onto the cornea.
A cobalt blue light is useful in assessing fluorescein dye tests. A direct ophthalmoscope can have a lens that is rarely used retrofitted with a cobalt blue filter (http://www.welchallyn.com/). Alternatively, an inexpensive cobalt blue filter that covers a standard penlight can be ordered from a human ophthalmic supply catalog (Bernell: 1-800-348-2225; Item ALPENF <www.bernell.com/product/140/2>).
Performing Culture and Cytology in the Field
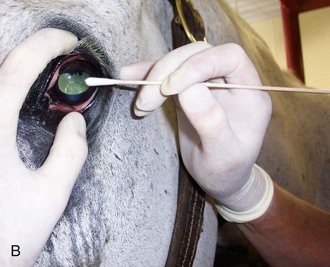
Supplies for culture and cytology should be packed in the ambulatory vehicle at all times (Box 2-5). The sample collection process can be paired with all the other procedures that are appropriate to the case, including digital imaging, dye tests, more extensive débridement of the lesion, and instillation of a subpalpebral lavage (SPL) system. Good planning of the sequence of sampling, imaging, and treatment will allow all procedures to be done quickly with minimal sedation.
Preparation for Diagnostic Sampling in the Field
Culture Sampling and Handling
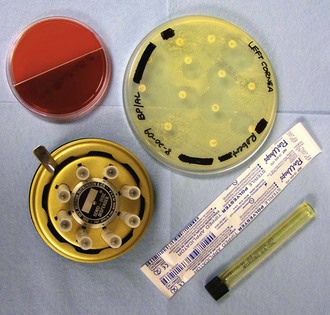
Antibiotic sensitivity testing can be referred to a reference lab or performed in-house using the Kirby-Bauer disk diffusion method of applying antibiotic impregnated disks to agar that has been plated with diluted colonies of bacteria. A sensitivity wheel should be designated for ophthalmic testing and filled with disks impregnated with the antibiotics commonly used to treat corneal infections (Fig. 2-10).
Samples plated on Sabouraud agar should be checked daily for fungal colony growth. Plates with growth can be sent off to a reference laboratory for sensitivity analysis but results may not be received in time to influence therapy choice. Please see Chapter 5 for more information on choice of therapy based on culture results.
Cytology Sampling and Handling
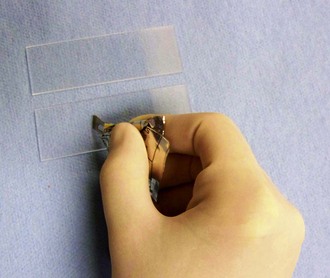
The cytology samples can be stained with Diff-Quik stain as soon as they are air dried (Fig. 2-12). They do not need any fixative or other prestaining preparation. Diff-Quik stain is a Romanovsky stain that yields excellent detail of cellular elements and is effective for staining cocci, most large and small rods, some bipolar rods, and most fungal hyphae.
Interpreting Ocular Cytology Samples
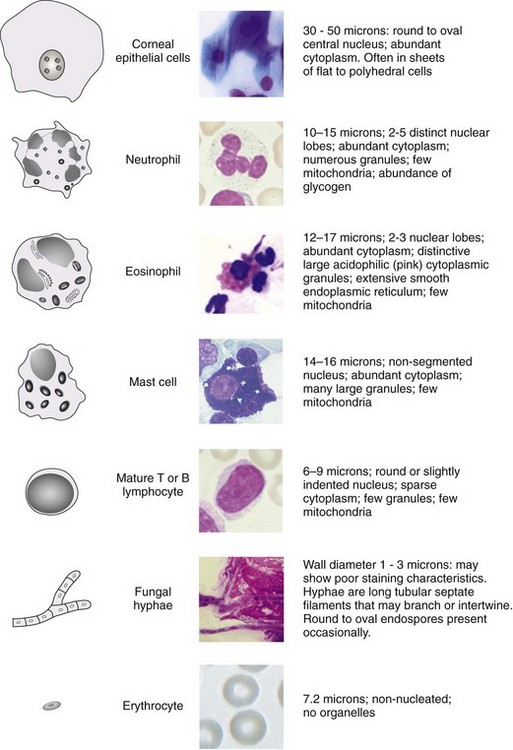
Only a limited number of cell types will be found in corneal samples, and the list of “other elements” commonly present in cytology samples from ulcers is short. With a little practice, typical findings can be easily discerned by equine practitioners and veterinary technicians (Fig. 2-13).
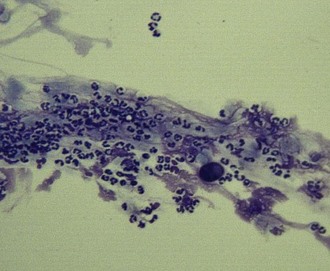
Scrapings of healthy cornea should contain nothing but sheets of epithelial cells (Fig. 2-14). Cells from the superficial layers are flattened with large amounts of blue cytoplasm and central basophilic nuclei. Cells from intermediate layers are more polyhedral, while cells from basal layers are more cylindrical and round and stain more darkly, showing less cytoplasm. Normal corneal cells do not contain bacteria and are exfoliated in sheets. Individual cells that are flat in contour may roll up into tight scrolls on the slide and must not be mistaken for hyphae or foreign bodies.
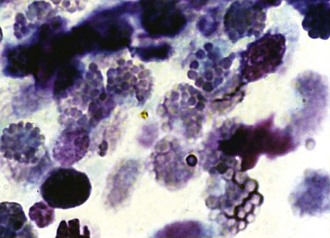
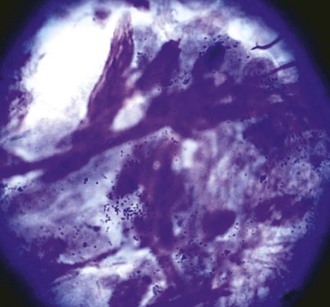
Scrapings from corneas with a cellular inflammatory response will show an infiltrate. The most common finding is a suppurative infiltrate, where neutrophils with or without toxic changes dominate the response (Figs. 2-15 and 2-16). A small number of lymphocytes, monocytes, or plasma cells may also be present. Although a suppurative infiltrate is not pathognomonic for infection, it is highly suggestive of it. Occasionally a corneal sample will show an eosinophilic infiltrate. Presence of eosinophils or basophils is abnormal and suggests either acute allergic hypersensitivity or eosinophilic keratitis (Fig. 2-17). Some fungal infections incite a granulomatous infiltrate consisting of epithelioid macrophages and giant cells. If hemorrhage has occurred due to the disease process or sampling trauma, red blood cells will be present in large numbers.
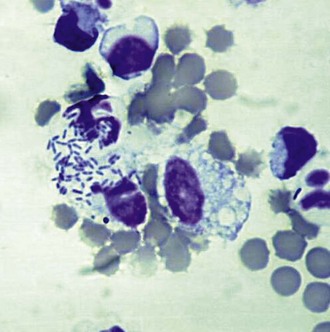
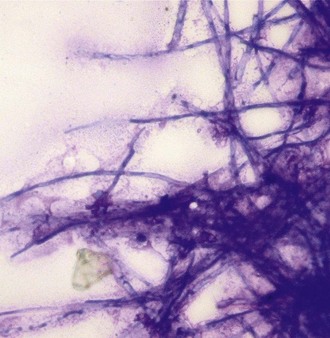
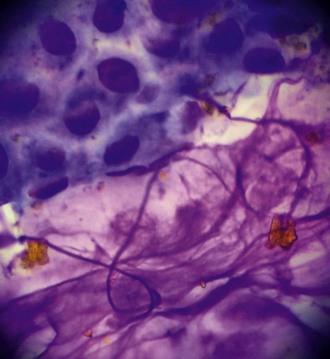
The presence of even a few fungal hyphae in a cytology sample is indicative of mycotic keratitis. Hyphae are slender, septate, branching, or linear structures. Most fungal hyphae stain well with Romanovsky stains and can be seen as a tangle of spaghetti-like densities interspersed with native epithelial cells (Fig. 2-18). Care must be taken to differentiate “scrolled” epithelial cells and long, thin strands of necrotic cellular debris from fungal hyphae (Fig. 2-19). The presence of fungal elements is always significant in ulcer samples, but the absence of hyphae does not rule out infection; hyphae may be present in corneal layers deep to the sampling site.
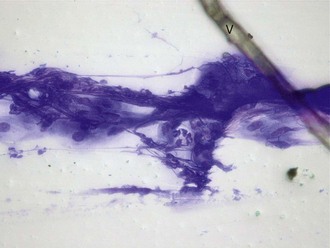
Other noncellular elements that may be seen on corneal cytology include intracytoplasmic melanin granules (dark green to black granules found in cells scraped from the limbal region or from pigmented areas [Fig. 2-20]), vegetative foreign bodies (Fig. 2-21), and mineralized crystals. The latter may stain light blue with Diff-Quik and may correlate with deposits of calcium in the subepithelial layers of horses with stromal keratopathies.3
Treatment Choices Based on Corneal Cytology
Rational therapeutic choices will follow as the cytology results are correlated with the clinical conditions described in Chapter 5 and the dominant infectious agents are identified. Most of the time, cytology will aid in choosing antiinfective, antiinflammatory, and antiprotease therapy, but in cases of suspected indolent ulcers, verification of the absence of cellular reaction or infectious elements is a critical step that must precede deeper débridement of a chronic lesion.
Other Ocular Cytology Indications
Cytology may also be indicated in tissues other than cornea. Samples can be taken from bulbar, palpebral, or nictitans conjunctiva that is inflamed, infected, or abnormal in appearance and analyzed as described earlier. Conjunctival samples may contain goblet cells in addition to the cellular and noncellular findings described in cornea. These cells have eccentric nuclei and pale blue cytoplasm (Fig. 2-22). A lymphocytic or eosinophilic infiltrate may be apparent in cases with allergic inflammation. Material can be collected from the tarsal margin to analyze cases of chalazion or meibomianitis.
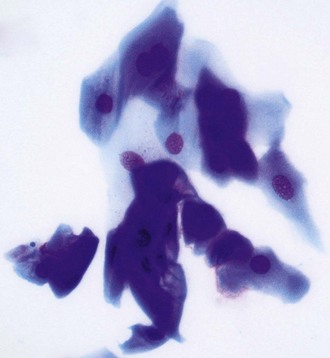
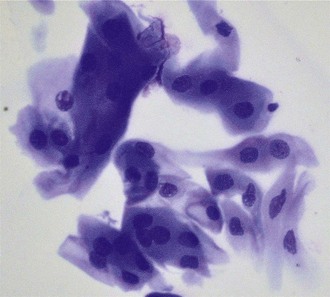
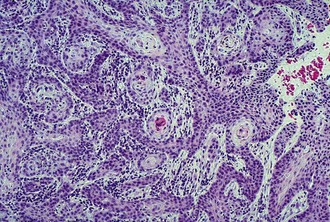
Lesions that are suspect for adnexal or corneal neoplasia can be analyzed by cytology samples of cells débrided from the surface, cells from impression smears, and cells from fine-needle aspirates. It is beyond the scope of the practice laboratory to analyze these specimens. They should be submitted to a reference laboratory. Most lesions suspicious for neoplasia should also be biopsied and fixed tissue samples submitted for histopathology (Fig. 2-23). Reports from clinical pathologists focus on several criteria when assessing dysplastic, anaplastic, or metaplastic cells for malignant potential3: