Postpartum Disorders
After giving birth, the female alpaca or llama is subject to a wide range of complications that are not unlike those seen in other large domestic species. Often, postpartum problems are evident at or immediately following parturition or may take hours to manifest. In the immediate postpartum period, the female should be monitored for normal maternal behavior toward the cria. Postpartum disorders may impede the female’s ability to develop the necessary dam–cria bond and also may prevent her from standing, which would impede the cria’s ability to nurse. Other postpartum disorders may go completely unnoticed and may compromise the fertility of the female. In an intensive production system, alpacas and llamas are rebred relatively shortly after parturition. Therefore, clients should be educated about the importance of postpartum examination of females to address any problems promptly and to help preserve the future fertility or even the life of the animal. This chapter describes the protocol for postpartum evaluation and the most common problems encountered in the postpartum period.
Examination of the Postparturient Female
Alpacas and llamas have a very rapid uterine involution and are rebred within 2 to 3 weeks after parturition. Uterine involution is about 80% complete by day 10 of the postpartum period. Conception rate is comparable with that of nonparturient females by 15 to 21 days after parturition.1 Uterine involution and the postpartum conception rate are determined by events during parturition and in the immediate postpartum period. Clients should be educated to call for veterinary examination of the postpartum female 24 to 36 hours after parturition, even if everything seems to be normal. Urgent examination should be performed after dystocia, if the behavior or the health of the female or the cria appear abnormal or if the placenta is not passed within 3 hours of parturition.
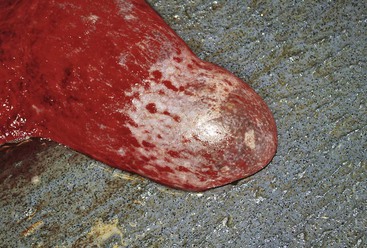
Routine postpartum evaluation of the female should include physical examination, body condition scoring, and evaluation of the mammary gland and the reproductive tract. Examination of the reproductive tract is often limited to the evaluation of the vulva, the vagina, and the cervix. The vulva should be examined for any external damage and parted to evaluate the degree of pain and bruising (Figure 26-1). A slight hyperemia is normal in the first 24 hours of the postpartum period. Some swelling of the vulva may be also evident but is not extreme. By days 2 to 3 of the postpartum period, all of the swelling should be completely resolved. The perineal area is cleaned, and the examination of the vagina is preferably performed using a large-tube speculum (2 to 5 centimeters [cm] in diameter, depending on the size). This technique allows better visualization of the vaginal cavity and the cervix. In the normal postparturient female, the cervix may display some edema and sometime areas of contusion, but not any obvious tears or bleeding. The vaginal cavity should be free from any laceration. Postpartum uterine discharge is often not seen at this examination or is minimal. Lochia in alpacas and llamas is very thick and will appear around days 3 to 5 of the postpartum period as a thick mucous discharge that is pink or whitish in color (Figure 26-2).
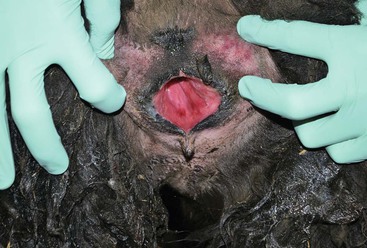
Figure 26-1 Examination of the Vulva and the Vestibular Area.
A slight hyperemia is normal in the first 24 hours in the postpartum period.
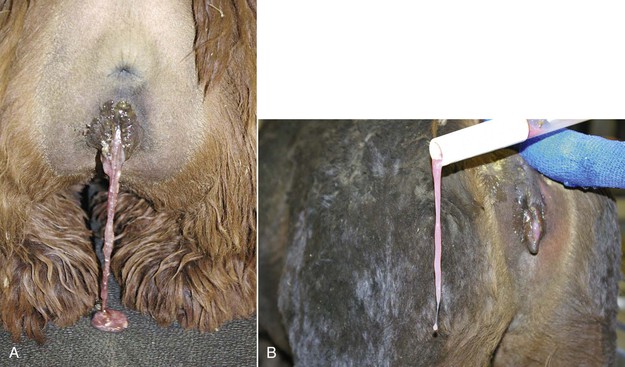
Figure 26-2 A, Postpartum lochia in alpacas and llamas is very thick and may be confused with partial retention of the placenta by inexperienced individuals. B, Lochia may hinder vaginal examination during vaginoscopy.
Emergency postpartum evaluation is warranted if the female rejects the cria or if obvious complications such as retained placenta, uterine prolapse, excessive vaginal bleeding, reluctance to stand, depression, poor appetite, postpartum colicky syndrome, or unexplained fever are present. In these situations, more advanced examination techniques, including complete blood cell count, blood biochemistry, transadbominal ultrasonography, and possibly abdominocentesis, should be considered. Referral to a specialty center or hospitalization may be indicated in many of these cases. Exploratory celiotomy or laparotomy may be the only way to reach a diagnosis.
Examination of the Placenta
The placenta should be emptied of its fluids and weighed. The normal weight of the placenta is generally about 9% to 12% of the cria’s birth weight. Alpaca and llama normal placenta weights vary between 0.6 to 1.6 kilogram [kg] and 0.8 to 1.8 kg, respectively. In one study on 398 alpacas, the mean placental weight was 876 ± 5.4 and 780 ± 32.3 grams (g) for live and dead crias, respectively.2 The same authors reported a significant increase in placental weight between ages 6 and 9 years, followed by a decrease after 10 years. However, placental efficiency was higher at age 6 years and at age 11 years. Placental weights in North America are significantly larger and vary between 0.8 and 2 kg. This also reflects the significantly higher birth weight observed in alpacas in North America.
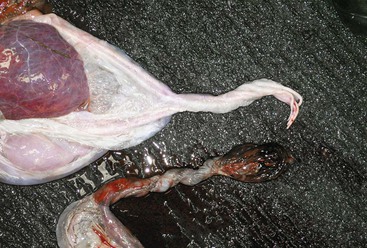
The placenta should be laid out flat and the chorionic surface examined for completeness and any lesions (necrosis, inflammation, or avillous areas). The tips of the horns should be verified, as small parts may be missing there (Figure 26-3). One way to examine the entire surface of the placenta is by filling it with water. The presence of an avillous line along the bifurcation is normal (Figure 26-4). The tip of the horn is an area of poor villous chorionic development (Figure 26-5). Suspicious areas should be sampled and submitted for histopathology, especially if the cria displays signs of septicemia or anoxia. The cervical start is generally not very well demarked compared with that of the equine because of the small tight cervix during pregnancy in camelids. It can be seen clearly in some cases after a cesarean section has been performed before rupture of the chorioallantoic sac. The chorionic surface may present areas of congestion or edema. Highly edematous and congested placentae are usually a feature of prolonged delivery or dystocia. Discoloration may be caused by an inflammatory process, but it is important to differentiate it from autolysis.
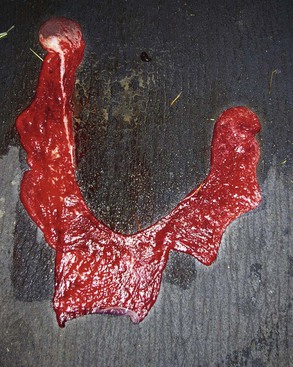
Figure 26-3 Normal placenta laid out in “Y” shape with the chorionic surface exposed. The left uterine horn is obviously larger than the right horn.
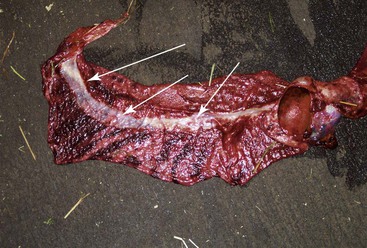
Figure 26-4 Avillous (arrows) Line Corresponding to the Uterine Bifurcation.
Note the congestion pattern of the chorionic surface. This is very typical in prolonged labor.
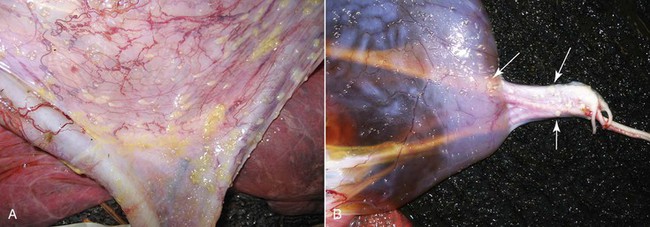
Following examination of the chorionic surface, the placenta may be inverted to examine the amnion. Unlike in the equine, the amnion cannot be spread and examined separated, as it closely adheres to the chorioallantoic sac (Figure 26-6). Small (1 to 3 millimeters [mm] in diameter), raised areas may be noticed on the amnion and the umbilical cord (Figure 26-7). These are normal amniotic plaques and have no functional significance. However, large plaques should be further investigated histopathologically. Vascularization of the amnion is not as marked in the camelid as in the equine but may be present. Single or multiple allantoic vesicles may be present on the allantoic surface (Figure 26-8). They may measure anywhere from a few millimeters up to 4 or 5 cm.
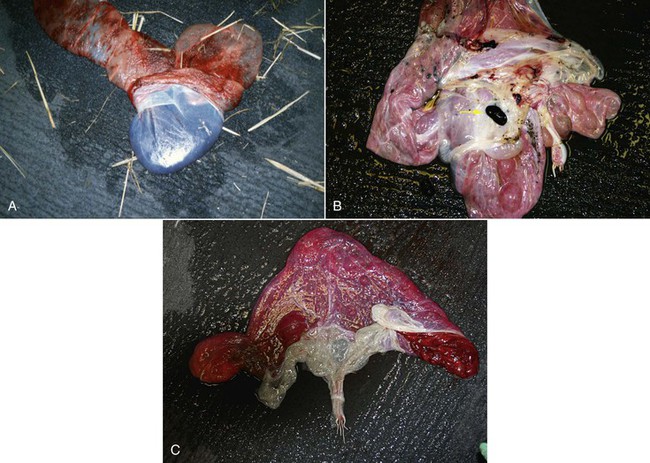
Figure 26-6 A, Amnion is firmly attached to the allantoic sac and cannot be spread and examined separately. B, Note the hippomane (arrow). C, Umbilical cord on the amniotic side.
Hippomanes (also called allantoic calculi) are often present in the allantoic cavity. These may number 1 to 4 and vary in size from a few millimeters to 4 cm. They are soft and flattened and vary in color from tan to dark brown. Hippomanes are composed of lipids, cellular debris, degenerated blood cells, and mineralized material.
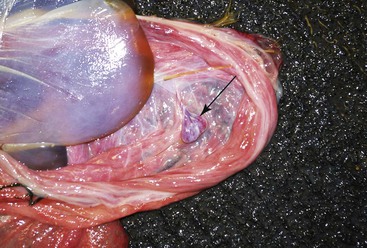
The umbilical cord should be examined closely for any abnormality. The most common abnormalities of the umbilical cord are funisitis, edema, or abnormal venous dilation (Figure 26-9). Remnants of the yolk sac are a common finding.
Retained Fetal Membranes (Retained Placenta)
Detachment of the placenta from the endometrium generally occurs rapidly, and the typical female camelid will expel the placenta shortly (less than 60 minutes) after parturition. Fetal membranes are considered retained if not expelled within 3 to 6 hours of delivery of the cria.3 Retention of the fetal membranes beyond 6 hours is commonly seen following dystocia or delivery by cesarean section. Placental retention may also be induced by excessive use of large doses of oxytocin and by indiscriminate postpartum lavage.
Contrary to common belief, retained placenta does not immediately put the female camelid at risk of endotoxemia, and the condition generally resolves within 24 to 48 hours. Females with retained placenta and systemic signs should be examined for peritonitis. Transcutaneous abdominal ultrasonography is indicated in the examination of these females. Uterine tear with passage of the placenta into the abdominal cavity has been described as a cause of peritonitis.4
Treatment options include administration of oxytocin (5 to 7.5 international units [IU] in alpacas, and 10 IU in llamas) intramuscularly every 4 to 6 hours in alpacas and llamas. Continuous-rate infusion of oxytocin in lactated Ringer solution given intravenously (IV) may be an alternative option if a venous catheter is already in place. Vaginal examination should be performed to determine the extent of cervical dilation and to assess for the presence of cervical edema, which may be preventing passage of the fetal membranes. Failure of cervical dilation is often a problem when a cesarean section was performed before term to save a compromised dam and may be treated by topical administration of prostaglandin E (misoprostol) to the cervix (0.5 milligrams [mg]). Dilation should ensue within several hours. However, to prevent this problem, we always administer a luteolytic dose of prostaglandin F2-alpha (PGF2α) or its analogue preoperatively once the female is stabilized. Uterine lavage is commonly performed in mares with retained fetal membranes, but often this is not performed in camelids as a first treatment option.3 In cases of partial eversion of the uterine horn or severe spasm, distension of the allantoic sac may be a good solution if the placenta is still intact. This will allow the uterine horns to be distended and help the placenta to detach. To accomplish this, a foal nasogastric tube is introduced inside the allantoic cavity, and the placenta is held firmly around it while fluid (5 to 10 liters [L]) is pumped. The female is allowed to expel the fluid, and the placenta is tugged to see if it is free. In most cases, it takes one to two distensions to remove a placenta that is retained because of uterine horn eversion or placental edema.
Serum calcium levels should be assessed in cases of retained fetal membranes and any hypocalcemia corrected, if present. Additionally, the animal should be evaluated for the presence of uterine tears by using transabdominal ultrasonography, especially during a uterine lavage, if performed (Figure 26-10). The presence of the lavage fluid in the abdomen will be easily identifiable with ultrasound. Alternatively, abdominocentesis may be diagnostic for uterine tears.
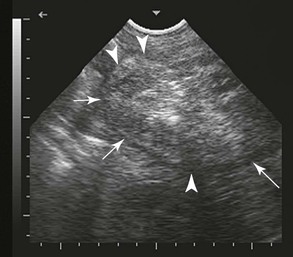
Figure 26-10 Transabominal ultrasonography of the uterine horn (arrows) with placenta (hyperechoic area) trapped at the tip.
Postpartum Hemorrhage
The hemorrhage may be noticed as a profuse discharge of bright red blood from the vagina. However, often the only warning sign may be intermittent discharge of blood clots from the vagina. The placenta may also serve as a compressing mass, and the full extent of bleeding may not be apparent until after placental delivery. In cases in which hemorrhage is intrauterine or intraabdominal, clinical signs may not be evident until extensive blood loss has occurred and circulatory shock has set in. The animal may be found dead several hours after parturition. Clinical signs of hemorrhage include colic signs, pale mucous membranes, tachycardia, muscle trembling, sweating, recumbency, and death.5
If the diagnosis of acute hemorrhage is made quickly, treatment may be attempted. Applying pressure to the site of hemorrhage is paramount and may be done by fashioning a large vaginal tampon of rolled cotton covered with brown gauze, sized appropriately for the female. Copious lubrication is applied to the tampon prior to insertion, or a topical antimicrobial ointment such as antibiotic-impregnated lanolin (500 g lanoline and 80 mg oxytetracycline). Circulating volume fluid replacement and plasma expansion therapy should be initiated as soon as possible (hypertonic saline followed by LRS). An intravenous jugular catheter is placed and fluid replacement therapy initiated, taking care not to overload the female with fluid. Camelid species are especially prone to developing pulmonary edema in the face of fluid excess.5 It is extremely important to keep the female calm while dealing with this emergency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree