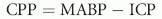Chapter 145 Postcraniotomy Management
• Imaging studies ideally are performed immediately following surgery, while the patient is still under anesthesia.
• After anesthetic recovery, animals usually are monitored in a critical care area for signs of neurologic deterioration or other complications such as pneumonia for at least 48 hours.
• Oral food and water are withheld until the animal regains swallowing function, which may take a number of days.
• Maintaining cerebral blood flow is essential during the recovery phase following intracranial surgery.
POSTOPERATIVE MANAGEMENT
Hydration
where CPP = cerebral perfusion pressure, MABP = mean arterial blood pressure.19,20 For CPP to remain constant, the effects of increased ICP on blood flow to the brain must be reciprocated by increases in systemic blood pressure. CPP is a determinant of cerebral blood flow but is not always equivalent. In many instances, however, CPP is a reasonable reflection of cerebral blood flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree